Abstract
A series of large-scale randomised controlled trials have demonstrated the effectiveness of lifestyle change in preventing type 2 diabetes in people with impaired glucose tolerance. Participants in these trials consumed a low-fat diet, lost a moderate amount of weight and/or increased their physical activity. Weight loss appears to be the primary driver of type 2 diabetes risk reduction, with individual dietary components playing a minor role. The effect of weight loss via other dietary approaches, such as low-carbohydrate diets, a Mediterranean dietary pattern, intermittent fasting or very-low-energy diets, on the incidence of type 2 diabetes has not been tested. These diets—as described here—could be equally, if not more effective in preventing type 2 diabetes than the tested low-fat diet, and if so, would increase choice for patients. There is also a need to understand the effect of foods and diets on beta-cell function, as the available evidence suggests moderate weight loss, as achieved in the diabetes prevention trials, improves insulin sensitivity but not beta-cell function. Finally, prediabetes is an umbrella term for different prediabetic states, each with distinct underlying pathophysiology. The limited data available question whether moderate weight loss is effective at preventing type 2 diabetes in each of the prediabetes subtypes.
Keywords: prediabetes, impaired fasting glucose, impaired glucose tolerance, weight loss, fibre protein, Mediterranean, low-carbohydrate, very low energy diets
1. Introduction
The Current Paradigm for Type 2 Diabetes Prevention
Current guidelines for the prevention of type 2 diabetes (T2D) in people at high risk are based around achieving moderate weight loss (3–7% weight loss) via dietary change and increasing physical activity [1]. In each of the major diabetes prevention trials (Table 1), dietary advice (for those categorised as overweight) was to lower fat intake to achieve a modest calorie deficit, and to increase physical activity [2,3,4,5,6]. Other dietary changes in some trials included increasing fibre and limiting intake of saturated fat and added sugar [2,6].
Table 1.
Brief description of the dietary and lifestyle changes included in the major type 2 diabetes prevention trials.
| Name of Study | Intervention |
|---|---|
| The Finnish Diabetes Prevention Study (FDPS) (2) | Aim for 5% weight loss, fat <30% kcal intake, saturated fat <10% kcal intake, fibre >15 g per 1000 kcal, PA: 30 min/day. |
| U.S. Diabetes Prevention Program (DPP) (3) | Aim for 7% weight loss, fat <25% kcal intake, PA: 150 min/week. |
| The Da-Qing Impaired Glucose Tolerance (IGT) and Diabetes Study (4) | High-carbohydrate and low-fat diet, ↑ PA by 12 units/day. Aim for 23 kg/m2 if BMI > 25 kg/m2. |
| Japanese Diabetes Prevention Trial (5) | Reduce BMI to 22 kg/m2. Dietary advice individualised, ↓ fat intake (<50 g/day), portion size, alcohol intake, ↓ eating out. PA: 30–40 min/day. |
| Indian Diabetes Prevention Study (6) | Avoid simple sugars and refined carbohydrate, fat <20 g/day, ↑ fibre. PA: 30 min/day. |
BMI: body mass index; PA: physical activity; ↑: increased; ↓: decreased.
These interventions (Table 1) were able to prevent up to two thirds of cases of T2D over the course of the trials, which were 3–6 years in length, with sustained risk reduction compared to the control groups for up to 20 years after the end of the trials [7]. However, within 15 years of the end of the trial, the majority of participants still developed T2D [8]. There are likely several reasons for this including insufficient weight loss and weight regain, a lack of effect of the intervention on beta-cell function, and differences in response depending on the subtype of prediabetes. Given the growing prevalence of prediabetes and T2D worldwide, addressing these issues will be important in helping prevent T2D in more people over the long term. This article will review the evidence to date and make recommendations for future research.
2. Interventions to Promote Weight Loss and Weight Loss Maintenance
Overall, these trials and supporting data show that weight loss is the primary driver of T2D risk reduction in people who are overweight [9]. In the US DPP, there was a 96% reduction in risk comparing the 90th against the 10th percentile of weight loss, [9] with each kg of weight loss associated with a 16% reduction in risk [9]. Individual dietary components of these interventions, such as increasing fibre and reducing saturated fat, to date have shown comparatively minimal effect in overweight people compared to simply losing weight [9,10,11], but might play a bigger role in risk reduction for relatively leaner individuals [4,6].
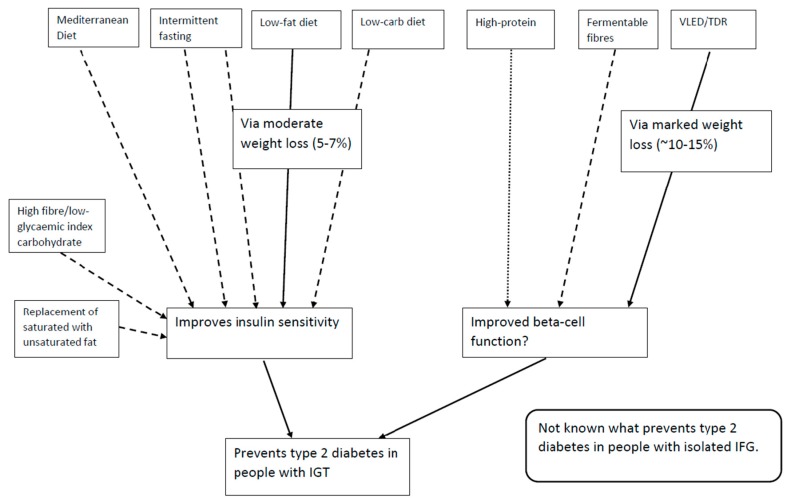
If weight loss is the primary driver of T2D risk reduction, it is possible that other dietary interventions which can lead to >5% weight loss will be as effective as the current guidelines in preventing T2D (Figure 1). Furthermore, long-term weight loss maintenance is more likely to be achieved with a diet that a person enjoys and can stick to. Offering a choice of dietary approach to the patient is therefore important.
Figure 1.
Current dietary strategies shown to improve (full line) or some evidence of improvement (dashed line) and potential for improvement (dotted line) for the two primary pathophysiological defects in the development of type 2 diabetes. IFG: impaired fasting glucose; IGT: impaired glucose tolerance; TDR: total diet replacement; VLED: very low energy diet (<800 kcal/day).
2.1. Low-Carbohydrate Diets
Data from meta-analyses show that low-carbohydrate diets (the definition can vary but typically under 30% energy from carbohydrate) are at least as good as low-fat diets (<30% of total energy intake from fat) at promoting weight loss [12,13]. Importantly, drop-outs from all weight loss trials are in the order of 35–50% [13,14] and the key factor in whether a participant finishes a trial is whether they like and are able to stick to the lifestyle change.
There are other aspects of low-carbohydrate diets which could theoretically help prevent the development of T2D in addition to weight loss. Glucotoxicity is defined as the physiological and eventually irreversible β-cell damage caused by chronic exposure to supraphysiological glucose concentrations [15]. Human islets which are experimentally exposed to high concentrations of glucose (11 mmol/L) show reduced insulin biosynthesis, reduced insulin secretion in response to elevated glucose concentrations, and increased rates of insulin release in the presence of low glucose, all of which are characteristics of insulin secretion in vivo in the prediabetic state [16]. Some of these effects can be reversed, depending on the duration of exposure [17]. It is important to note that in these experiments the exposure to hyperglycaemia was continuous and may not reflect daily in vivo glucose profiles. Nonetheless, in mildly prediabetic subjects, blood glucose concentrations may remain over 7.8 mmol/L over an extended post-prandial period [18,19]. It has also been observed that typical consumption of three meals per day (notwithstanding additional snacking or carbohydrate-containing drinks) means that people can spend more than half the day in a post-prandial or post-absorptive state [20]. Marked carbohydrate restriction (~8% of total energy from carbohydrates) is able to rapidly lower post-prandial glucose concentrations in people without T2D [21]. Therefore, although it remains to be tested in prospective trials, dietary interventions which lower post-prandial glucose in people with impaired glucose tolerance (IGT) may be particularly effective at preventing T2D via protecting beta-cell function. The degree of carbohydrate restriction which can meaningfully lower post-prandial glucose concentrations remains to be determined.
Low-carbohydrate diets could also theoretically protect the beta-cell by reducing insulin demand. It has been proposed that beta-cell exhaustion occurs due to the constant demands of secretion induced by frequent and prolonged episodes of hyperglycaemia [16]. In human islets exposed to continuous hyperglycaemia the use of diazoxide to inhibit insulin secretion helps to prevent hyperglycaemia-induced damage to the islets, and preserves their capacity to synthesise and release insulin [22]. On the other hand, the short-term human data that currently exists suggests that a high-fat diet could impair beta-cell function [23], with in vitro data suggesting saturated fat may be particularly deleterious in this regard [24]. Given the importance of beta-cell function to T2D prevention, this should be urgently studied, and long-term follow-up data is needed.
Ectopic fat is more strongly associated with T2D than BMI, and might play a role in the development or exacerbation of insulin resistance and beta-cell dysfunction [25]. Despite claims that low-carbohydrate diets might help lower ectopic fat deposition, the evidence is currently unclear. Marked restriction of carbohydrates to under 30 g/day appears to lower intrahepatic triglyceride in the absence of weight loss, but it is not clear whether this is due to the high protein content of the interventions [26,27], with the type of fat [28] likely playing an additional role.
2.2. Mediterranean Dietary Pattern
Mediterranean diets have also been shown to help weight loss [29], though again are not superior to any other approach. However, the Mediterranean diet may also help prevent T2D independent of weight loss.
A pre-planned secondary analysis of the PREDIMED trial showed that a Mediterranean-style diet supplemented with nuts or extra virgin olive oil helps prevent T2D compared to the control diet [30]. This result was striking as the risk reduction occurred in the absence of weight loss. As noted previously, there were very few differences between the control and intervention diets, and any effects appear to be due to the addition of 30 g of nuts or extra virgin olive oil as opposed to regular olive oil [31]. Due to discrepancies in the randomisation procedure, the primary findings of PREDIMED were withdrawn and rewritten, though the outcomes remained materially unchanged. The same appears to be true of the reduction in T2D incidence [32]. There is also limited understanding of the mechanisms of a Mediterranean-style dietary pattern on T2D prevention and these are likely multi-component. Putative mechanisms include improvements in insulin sensitivity via a reduction in inflammation [33], and beneficial effects of fatty acids [34] and phenolic compounds [35] on the beta-cell. A Mediterranean-style diet may also help lower liver fat [36].
Given the proposed beneficial properties of a Mediterranean diet on T2D independent of calorie restriction, combining a weight-reducing diet with a Mediterranean-style diet would be a useful approach.
2.3. Intermittent Fasting
Intermittent fasting (IF) describes diets which limit calorie intake on certain days (varying in number and whether consecutive or separate), or at certain times [37] (Table 2). Due to the variation in protocols used, interpreting the evidence for these is challenging. Overall, such trials result in the loss of 2.5–9.9% body weight, comparable to weight loss from continuous energy restriction [37]. Drop-out rates are also comparable to other weight loss trials, reaching 40%. The literature on time–restricted feeding (TRF) varies by the time period allocated for energy consumption, and the majority were studies on Ramadan fasting or trials where weight loss was not an aim. Unsurprisingly, these trials show little or only modest changes in weight [38].
Table 2.
Summary and brief description of types of intermittent fasting (IF). Adapted from [37] and used with permission.
| Type of Intermittent Fasting | Description |
|---|---|
| Alternate day fasting | Alternating feast (ad libitum intake) and fast days (≤25% of energy needs) |
| Time-restricted fasting | Eating only during certain time periods (i.e., 8 h), then fasting for remaining hours of the day |
| Periodic fasting | Fasting for up to 24 h once or twice a week with ad libitum intake on the remaining days |
There is also interest in proposed specific benefits of IF, particularly on insulin sensitivity. In general, the effect of alternate-day or period fasting on insulin sensitivity is unclear, with some trials showing benefit and others an adverse effect [37]. The majority of data on TRF comes from animal studies, and human studies have largely lacked the robust methodology required to study insulin sensitivity under controlled conditions [38]. However, a recent small but well-controlled crossover trial [39] found that restricting energy intake from 6:00 a.m. to 2:00 p.m. for five weeks in obese males with prediabetes improved insulin sensitivity and increased fat oxidation compared to usual energy intake. This study only included eight people so larger studies involving both genders are required. Nevertheless, given that weight loss and its maintenance are the cornerstones of T2D prevention, IF/TRF diets increase the range of options available to people to achieve weight loss. Any additional physiological benefits remain to be confirmed.
2.4. Very-Low-Energy-Diets
A very low-energy diet (VLED) is defined as a diet with <800 kcal per day. VLEDs consistently produce greater weight loss than other diets [40,41], and contrary to conventional wisdom, rapid weight loss does not increase the likelihood of weight regain [40,41,42]. Very-low-energy diets are associated with better weight-loss maintenance than moderate energy-restricted diets for up to five years of follow-up [42], and greater weight loss [9] and greater weight-loss maintenance [43] are the key drivers of T2D risk reduction.
In addition, a series of physiological trials of varying length have shown that VLEDs can achieve normoglycaemia in people with established T2D via improvements in hepatic and peripheral insulin sensitivity, and via restoration of the first-phase insulin response [44,45,46,47,48,49,50]. Both the amount of weight loss and the rate of weight loss (caloric restriction per se) appear to be independent drivers of the glucose-lowering effect: Better glucose control and insulin sensitivity are observed in people losing 12% of weight on 400 kcal/day compared with 1000 kcal/day [49]. Seven days of 400 kcal/day leads to negligible weight loss, but restores beta-cell function in people with T2D [45]. This confirms findings from bariatric surgery that improvements in glycaemic control occur before any significant weight loss. People with prediabetes already have hepatic and peripheral insulin resistance and impaired beta-cell function [51], and would likely benefit in the same way from these types of interventions [52].
The Finnish Diabetes Prevention Study included a VLED as part of the lifestyle intervention [53], but this was only used if participants had not met their weight target. Therefore, a VLED-type approach has not been tested in the prevention of T2D. The cost of delivering resource-intensive one-to-one support in a long-term VLED intervention, such as the recent type 2 diabetes remission trial DiRECT [54], might be prohibitive currently. The national diabetes prevention programme in the UK—the NHS diabetes prevention program [55]—is delivered in groups and with less frequent support, and is therefore less resource-intensive.
3. Interventions to Improve Beta-Cell Function
The two primary defects causing the development of T2D are insulin resistance and beta-cell dysfunction [56] (Figure 1). Moderate weight loss via a low-fat diet (<30% total energy from fat) and moderate physical activity improves insulin sensitivity [57,58,59], which probably helps protect the beta-cell over time via limiting compensatory hyperinsulinaemia [60]. However, post-hoc data from the Finnish and US diabetes prevention studies showed no independent effect of the intervention on the absolute insulin secretory response once changes in insulin sensitivity were taken into account [57,59]. This is important because the seminal event in the conversion from prediabetes to T2D is beta-cell failure [61].
The qualitative aspects of beta-cell function in both the fasting and post-prandial state are not fully understood. However, the physiological importance of the pulsatile and first-phase insulin response is clear.
3.1. Pulsatile Insulin Secretion
It is well established that insulin is secreted by the beta-cell in a pulsatile pattern in the fasting and post-prandial states in approximately 5-min cycles [62]. The pulsatile pattern may help to prevent de-sensitisation of insulin receptors from continuous exposure to insulin. Indeed, insulin infused in a constant versus pulsatile pattern leads to increased endogenous glucose production [63]. The amplitude and frequency of insulin pulses are lost in the prediabetic state [62], and in minimally glucose intolerant relatives of people with T2D [64].
3.2. First-Phase Insulin Response
Following a rapid increase in blood glucose, the beta-cells respond with an immediate, pronounced release of insulin (first phase) which serves to rapidly suppress hepatic glucose output [65,66], followed by a second phase which promotes glucose uptake until normalisation of glucose concentrations is achieved. This biphasic insulin secretion can be clearly observed following intravenous infusion of glucose, but is less well defined following an oral glucose load [65].
The physiological importance of the first-phase insulin response is demonstrated by studies in which its experimental suppression results in impaired suppression of hepatic glucose output [67], and higher maximal glucose concentrations [68]. When combined with insulin resistance, these defects lead to prolonged hyperglycaemia which may last for several hours [68]. Conversely, experimental restoration of the absent early insulin response normalises glucose tolerance without increasing the overall insulin demand [68]. Therefore, an impaired first-phase insulin response could (1) place more demand on the beta-cell to control postprandial glucose concentrations (via the second phase); and (2) contribute to a toxic metabolic and hormonal milieu which exacerbates the underlying pathophysiology. Preserving (or restoring) the first-phase insulin response should therefore be a focus of T2D prevention [69].
Precise measurement of the first-phase or acute insulin response requires a hyperglycemic clamp, a frequently sampled intravenous glucose tolerance test, C-peptide deconvolution or the use of tracers [70], which to date have been used infrequently. The following section highlights the current evidence base for dietary interventions on beta-cell function.
3.3. Effect of Diet on Beta-Cell Function
The effect of dietary interventions in insulin pulsatility is unclear as there is a near absence of interventional studies which have measured this [71]. There is a limited but growing body of research on the effects of diets and nutrients on the first-phase insulin response.
As described above, marked energy restriction and/or weight loss can restore the first-phase insulin response in people with established T2D [45,46,50], but moderate weight loss or physical activity does not appear to [57,58,72,73,74]. The mechanism is currently unclear, but might be due to reducing ectopic fat [50], reducing glucotoxitciy [15,16] and allowing the beta-cell to rest [75].
There is limited data suggesting that dietary fibre may improve beta-cell function [76,77,78,79] (Figure 1), but this has not been studied extensively. The effect may be via increasing glucagon-like peptide-1 (GLP-1) [79] or by a direct effect of short-chain fatty acids (produced by colonic fermentation of fibre) on the beta-cell [80]. The type of fibre or combinations of fibres which are most effective is not clear currently.
Protein may potentiate insulin secretion via the incretin hormones gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) [81]. In addition, amino-acid-specific insulin secretion may also play a role. Amino acids act alone or synergistically with glucose to potentiate the release of insulin [82], and while amino-acid-stimulated insulin secretion (AAIS) shares some pathways with glucose-stimulated insulin secretion (GSIS), some pathways are distinct [83]. There are some acute and chronic studies which have estimated insulin secretion via an oral glucose tolerance test which suggest that protein foods may help the secretion of insulin post-prandially [84,85], but further studies are required to confirm this. A note of caution is required given findings that a higher-protein diet may ameliorate weight-loss-induced improvements in insulin sensitivity compared to a lower-protein diet [86], and the clinical significance of this long-term is unknown.
Eight weeks of a high-carbohydrate (CHO) (55% CHO; 27% fat) diet in overweight men and women with prediabetes improved the beta-cell response to glucose compared to a lower-CHO, higher-fat diet [87]. (43% CHO; 39% fat). However, two weeks of a high-fat, moderate-carbohydrate diet (43% fat and 40% carbohydrate) had no effect on the acute insulin response in healthy normoglycaemic males compared to a low-fat, high-carbohydrate diet (25% fat and 56% carbohydrate) [88]. The fibre content of the meals in these studies differed, which may have played a role in modulation of beta-cell function.
There is therefore currently not sufficient evidence to make nutrient-based recommendations to improve beta-cell function (Figure 1). Given the importance of beta-cell function to the pathogenesis of T2D, this area of research should be prioritised.
4. Interventions Which Target the Prediabetic Subtype
Prediabetes is an umbrella term for at least two conditions: impaired fasting glucose (IFG) and impaired glucose tolerance (IGT). IFG is defined by the WHO as a fasting plasma glucose (FPG) of >6.1 mmol/L, while IGT is a glucose concentration two hours after a 75 g oral glucose tolerance test of 7.8–11.0 mmol/L [89]. A person can have elevated FPG with normal 2-h glucose concentration (isolated-IFG), or a normal FPG with elevated two-hour plasma glucose (isolated IGT) or with both FPG and two-hour glucose elevated (IFG/IGT).
The current guidelines for T2D prevention are based on the results of lifestyle interventions which were all carried out in populations with IGT (with or without IFG) [2,3,4,5,6] (Figure 1). A T2D prevention study in Japan [90] also included a sub-group with isolated IFG (I-IFG) and found that the intervention did not lower the risk of T2D in this group. The adjusted hazard ratio (HR) was 1.17 (95% confidence interval (CI), CI 0.50–2.74) in the I-IFG group compared to 0.41 (0.24–0.69) in the IFG/IGT group. However, the incidence of T2D in the I-IFG group was six times lower than the incidence in the IFG/IGT group, suggesting that this group was at relatively low baseline risk [90].
The D-Clip study also included people from all subtypes of prediabetes and also found non-significant risk reduction in the I-IFG group (12%) compared to I-IGT (31%) and IFG/IGT (36%) groups [91]. Moreover, the study design included a plan for the introduction of metformin in participants at highest risk of conversion to diabetes at ≥4 months of follow-up. The proportion of people with I-IFG requiring metformin was higher (77%) than the I-IGT group (51%) [91]. This might explain the absence of protective effect.
A peer-support T2D prevention study in India [92] also found no risk reduction in the I-IFG group and a post-hoc analysis of the Finnish Diabetes Prevention Study found differences in the effect of the intervention on fasting versus two-hour glucose concentrations, which also differed between people with IFG/IGT and people with I-IGT [93]. It is important to note that none of these studies had an a priori hypothesis to test whether the effect of the intervention was different between prediabetes subtypes, and none were powered to do so. However, the evidence is consistent in suggesting that the prediabetes subtype could mediate the effectiveness of lifestyle change to prevent T2D. The prevalence of I-IFG may reach 10% of the population [94], and approximately one fifth of people develop T2D via elevated FPG with a normal two-hour glucose concentration [95]. Understanding the effect of lifestyle in preventing T2D via I-IFG is therefore an important public health question (Figure 1).
The distinct differences in underlying pathophysiology may help to explain why lifestyle change could affect progression to T2D to different degrees. IFG is characterised by marked hepatic insulin resistance, elevated hepatic glucose output, but normal muscle insulin sensitivity [96]. Conversely, in IGT there may be mild hepatic insulin resistance and marked muscle insulin resistance. There are also differences in insulin secretion—in IGT there are defects in the first and second-phase insulin responses, while in IFG the first-phase response is defective, but the second-phase remains intact [51].
Research into the effect of diet on the underlying pathophysiology of T2D will provide insight into the larger question of preventing T2D and also whether it is possible to optimise T2D prevention by targeting diet to the underlying pathophysiology.
5. Summary
The totality of evidence from diabetes prevention studies worldwide shows the importance of weight loss in the prevention of T2D. The dietary intervention used in these studies has been tested in multiple populations, ethnicities and settings, and represents the strongest evidence base currently. On the other hand, it is also true that the dietary intervention used in these interventions has not been compared to other diets. This work should be undertaken. This will not only increase choice for patients, and therefore potentially adherence, but may also reveal approaches which may be more effective than the current standard low-fat diet. It is also necessary to understand how diet affects the underlying pathophysiology. By identifying the mechanism of the effect of foods and nutrients on beta-cell function and tissue-specific insulin sensitivity, we could then design and test interventions which target the underlying physiological defects. This may be particularly relevant to prevention of T2D in people with isolated-impaired fasting glucose, in whom the effectiveness of current diabetes prevention programs has not been demonstrated.
Author Contributions
This review article was conceived of, and written entirely by N.D.G.
Funding
This research received no external funding.
Conflicts of Interest
N.D.G. has received grant or fellowship funding from Diabetes UK, Medical Research Council, Winston Churchill Memorial Trust, National Obesity Forum and Weight Watchers. N.D.G. has received payment from Ways of Eating™ for reviewing the content of an app to manage type 2 diabetes (Fixing Dad™), and from Oviva™ for evaluation of a technology-enabled, remote diabetes structured education programme.
References
- 1.National Institute for Health and Clinical Excellence . Preventing Type 2 Diabetes: Risk Identification and Interventions for Individuals at High Risk. National Institute for Health and Clinical Excellence; London, UK: 2012. NICE Guidelines (PH38) Updated: September 2017. [Google Scholar]
- 2.Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 3.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M., Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B., et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Kosaka K., Noda M., Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V. Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 7.Schwarz P.E., Greaves C.J., Lindström J., Yates T., Davies M.J. Nonpharmacological interventions for the prevention of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012;8:363–373. doi: 10.1038/nrendo.2011.232. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamman R.F., Wing R.R., Edelstein S.L., Lachin J.M., Bray G.A., Delahanty L., Hoskin M., Kriska A.M., Mayer-Davis E.J., Pi-Sunyer X., et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honsek C., Kabisch S., Kemper M., Gerbracht C., Arafat A.M., Birkenfeld A.L., Dambeck U., Osterhoff M.A., Weickert M.O., Pfeiffer A.F. Fibre supplementation for the prevention of type 2 diabetes and improvement of glucose metabolism: The randomised controlled Optimal Fibre Trial (OptiFiT) Diabetologia. 2018;61:1295–1305. doi: 10.1007/s00125-018-4582-6. [DOI] [PubMed] [Google Scholar]
- 11.Jebb S.A., Lovegrove J.A., Griffin B.A., Frost G.S., Moore C.S., Chatfield M.D., Bluck L.J., Williams C.M., Sanders T.A., RISCK Study Group Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: The RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am. J. Clin. Nutr. 2010;92:748–758. doi: 10.3945/ajcn.2009.29096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansoor N., Vinknes K.J., Veierød M.B., Retterstøl K. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2016;115:466–479. doi: 10.1017/S0007114515004699. [DOI] [PubMed] [Google Scholar]
- 13.Foster G.D., Wyatt H.R., Hill J.O., Makris A.P., Rosenbaum D.L., Brill C., Stein R.I., Mohammed B.S., Miller B., Rader D.J., et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010;153:147–157. doi: 10.7326/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahanty L.M., Riggs M., Klioze S.S., Chew R.D., England R.D., Digenio A. Maximizing retention in long-term clinical trials of a weight loss agent: Use of a dietitian support team. Obes. Sci. Pract. 2016;2:256–265. doi: 10.1002/osp4.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson R.P., Harmon J., Tran P.O., Tanaka Y., Takahashi H. Glucose toxicity in beta-cells: Type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–587. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 16.Eizirik D.L., Korbutt G.S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J. Clin. Investig. 1992;90:1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federici M., Hribal M., Perego L., Ranalli M., Caradonna Z., Perego C., Usellini L., Nano R., Bonini P., Bertuzzi F., et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: A potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes. 2001;50:1290–1301. doi: 10.2337/diabetes.50.6.1290. [DOI] [PubMed] [Google Scholar]
- 18.Hanefeld M., Sulk S., Helbig M., Thomas A., Köhler C. Differences in Glycemic Variability Between Normoglycemic and Prediabetic Subjects. J. Diabetes Sci. Technol. 2014;8:286–290. doi: 10.1177/1932296814522739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall H., Perelman D., Breschi A., Limcaoco P., Kellogg R., McLaughlin T., Snyder M. Glucotypes reveal new patterns of glucose dysregulation. PLoS Biol. 2018;16:e2005143. doi: 10.1371/journal.pbio.2005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monnier L., Colette C. Target for glycemic control: Concentrating on glucose. Diabetes Care. 2009;32:S199–S204. doi: 10.2337/dc09-S310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noakes M., Foster P.R., Keogh J.B., James A.P., Mamo J.C., Clifton P.M. Comparison of isocaloric very low carbohydrate/high saturated fat and high carbohydrate/low saturated fat diets on body composition and cardiovascular risk. Nutr. Metab. 2006;3:1–13. doi: 10.1186/1743-7075-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song S.H., Rhodes C., Veldhuis J.D., Butler P.C. Diazoxide attenuates glucose-induced defects in first-phase insulin release and pulsatile insulin secretion in human islets. Endocrinology. 2003;144:3399–3405. doi: 10.1210/en.2003-0056. [DOI] [PubMed] [Google Scholar]
- 23.Numao S., Kawano H., Endo N., Yamada Y., Konishi M., Takahashi M., Sakamoto S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur. J. Clin. Nutr. 2012;66:926–931. doi: 10.1038/ejcn.2012.58. [DOI] [PubMed] [Google Scholar]
- 24.Maedler K., Spinas G.A., Dyntar D., Moritz W., Kaiser N., Donath M.Y. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 25.Sattar N., Gill J.M. Type 2 diabetes as a disease of ectopic fat? BMC Med. 2014;12:1–6. doi: 10.1186/s12916-014-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mardinoglu A., Wu H., Bjornson E., Zhang C., Hakkarainen A., Räsänen S.M., Lee S., Mancina R.M., Bergentall M., Pietiläinen K.H., et al. An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell Metab. 2018;27:559–571. doi: 10.1016/j.cmet.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Browning J.D., Baker J.A., Rogers T., Davis J., Satapati S., Burgess S.C. Short-term weight loss and hepatic triglyceride reduction: Evidence of a metabolic advantage with dietary carbohydrate restriction. Am. J. Clin. Nutr. 2011;93:1048–1052. doi: 10.3945/ajcn.110.007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Della Pepa G., Vetrani C., Lombardi G., Bozzetto L., Annuzzi G., Rivellese A.A. Isocaloric Dietary Changes and Non-Alcoholic Fatty Liver Disease in High Cardiometabolic Risk Individuals. Nutrients. 2017;9:1065. doi: 10.3390/nu9101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito K., Kastorini C.M., Panagiotakos D.B., Giugliano D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab. Syndr. Relat. Disord. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 30.Salas-Salvadó J., Bulló M., Babio N., Martínez-González M.Á., Ibarrola-Jurado N., Basora J., Estruch R., Covas M.I., Corella D., Arós F., et al. PREDIMED Study Investigators. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appel L.J., Van Horn L. Did the PREDIMED trial test a Mediterranean diet? N. Engl. J. Med. 2013;368:1353–1354. doi: 10.1056/NEJMe1301582. [DOI] [PubMed] [Google Scholar]
- 32.Salas-Salvadó J., Bulló M., Babio N., Martínez-González M.Á., Ibarrola-Jurado N., Basora J., Estruch R., Covas M.I., Corella D., Arós F., et al. PREDIMED Study Investigators. Erratum. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2018;43:14–19. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bock M., Derraik J.G., Brennan C.M., Biggs J.B., Morgan P.E., Hodgkinson S.C., Hofman P.L., Cutfield W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE. 2013;8:e57622. doi: 10.1371/journal.pone.0057622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maedler K., Oberholzer J., Bucher P., Spinas G.A., Donath M.Y. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic beta-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 35.Maiorino M.I., Bellastella G., Petrizzo M., Scappaticcio L., Giugliano D., Esposito K. Anti-inflammatory Effect of Mediterranean Diet in Type 2 Diabetes Is Durable: 8-Year Follow-up of a Controlled Trial. Diabetes Care. 2016;39:e44–e45. doi: 10.2337/dc15-2356. [DOI] [PubMed] [Google Scholar]
- 36.Anania C., Perla F.M., Olivero F., Pacifico L., Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J. Gastroenterol. 2018;24:2083–2094. doi: 10.3748/wjg.v24.i19.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stockman M.C., Thomas D., Burke J., Apovian C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018;7:172–185. doi: 10.1007/s13679-018-0308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothschild J., Hoddy K.K., Jambazian P., Varady K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014;72:308–318. doi: 10.1111/nure.12104. [DOI] [PubMed] [Google Scholar]
- 39.Sutton E.F., Beyl R., Early K.S., Cefalu W.T., Ravussin E., Peterson C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018;27:1212–1221. doi: 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parretti H.M., Jebb S.A., Johns D.J., Lewis A.L., Christian-Brown A.M., Aveyard P. Clinical effectiveness of very-low-energy diets in the management of weight loss: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016;17:225–234. doi: 10.1111/obr.12366. [DOI] [PubMed] [Google Scholar]
- 41.Vink R.G., Roumans N.J., Arkenbosch L.A., Mariman E.C., van Baak M.A. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity. 2016;24:321–327. doi: 10.1002/oby.21346. [DOI] [PubMed] [Google Scholar]
- 42.Anderson J.W., Konz E.C., Frederich R.C., Wood C.L. Long-term weight-loss maintenance: A meta-analysis of US studies. Am. J. Clin. Nutr. 2001;74:579–584. doi: 10.1093/ajcn/74.5.579. [DOI] [PubMed] [Google Scholar]
- 43.Penn L., White M., Lindström J., den Boer A.T., Blaak E., Eriksson J.G., Feskens E., Ilanne-Parikka P., Keinänen-Kiukaanniemi S.M., Walker M., et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: Analysis of European Diabetes Prevention Study RCT. PLoS ONE. 2013;8:e57143. doi: 10.1371/journal.pone.0057143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henry R.R., Scheaffer L., Olefsky J.M. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1985;61:917–925. doi: 10.1210/jcem-61-5-917. [DOI] [PubMed] [Google Scholar]
- 45.Malandrucco I., Pasqualetti P., Giordani I., Manfellotto D., De Marco F., Alegiani F., Sidoti A.M., Picconi F., Di Flaviani A., Frajese G., et al. Very-low-calorie diet: A quick therapeutic tool to improve β cell function in morbidly obese patients with type 2 diabetes. Am. J. Clin. Nutr. 2012;95:609–613. doi: 10.3945/ajcn.111.023697. [DOI] [PubMed] [Google Scholar]
- 46.Jackness C., Karmally W., Febres G., Conwell I.M., Ahmed L., Bessler M., McMahon D.J., Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients. Diabetes. 2013;62:3027–3032. doi: 10.2337/db12-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sathananthan M., Shah M., Edens K.L., Grothe K.B., Piccinini F., Farrugia L.P., Micheletto F., Man C.D., Cobelli C., Rizza R.A., et al. Six and 12 Weeks of Caloric Restriction Increases β Cell Function and Lowers Fasting and Postprandial Glucose Concentrations in People with Type 2 Diabetes. J. Nutr. 2015;145:2046–2051. doi: 10.3945/jn.115.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley D.E., Wing R., Buonocore C., Sturis J., Polonsky K., Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1993;77:1287–1293. doi: 10.1210/jcem.77.5.8077323. [DOI] [PubMed] [Google Scholar]
- 49.Wing R.R., Blair E.H., Bononi P., Marcus M.D., Watanabe R., Bergman R.N. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17:30–36. doi: 10.2337/diacare.17.1.30. [DOI] [PubMed] [Google Scholar]
- 50.Lim E.L., Hollingsworth K.G., Aribisala B.S., Chen M.J., Mathers J.C., Taylor R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanat M., Mari A., Norton L., Winnier D., DeFronzo R.A., Jenkinson C., Abdul-Ghani M.A. Distinct β-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012;61:447–453. doi: 10.2337/db11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utzschneider K.M., Carr D.B., Barsness S.M., Kahn S.E., Schwartz R.S. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J. Clin. Endocrinol. Metab. 2004;89:2704–2710. doi: 10.1210/jc.2003-031827. [DOI] [PubMed] [Google Scholar]
- 53.Eriksson J., Lindström J., Valle T., Aunola S., Hämäläinen H., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Laakso M., Lauhkonen M., Lehto P., et al. Prevention of Type II diabetes in subjects with impaired glucose tolerance: The Diabetes Prevention Study (DPS) in Finland. Study design and 1-year interim report on the feasibility of the lifestyle intervention programme. Diabetologia. 1999;42:793–801. doi: 10.1007/s001250051229. [DOI] [PubMed] [Google Scholar]
- 54.Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [DOI] [PubMed] [Google Scholar]
- 55.National Health Service . NHS England Impact Analysis of Implementing NHS Diabetes Prevention Programme, 2016 to 2021. National Health Service; London, UK: 2016. [(accessed on 15 July 2018)]. Available online: https://www.england.nhs.uk/wp-content/uploads/2016/08/impact-assessment-ndpp.pdf. [Google Scholar]
- 56.Fonseca V.A. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S151–S156. doi: 10.2337/dc09-S301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Mello V.D., Lindström J., Eriksson J., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Sundvall J., Laakso M., Tuomilehto J., Uusitupa M. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: The Finnish Diabetes Prevention Study. Diabetes Care. 2012;35:211–217. doi: 10.2337/dc11-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitabchi A.E., Temprosa M., Knowler W.C., Kahn S.E., Fowler S.E., Haffner S.M., Andres R., Saudek C., Edelstein S.L., Arakaki R., et al. Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: Effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penn L., White M., Oldroyd J., Walker M., Alberti K.G., Mathers J.C. Prevention of type 2 diabetes in adults with impaired glucose tolerance: The European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342. doi: 10.1186/1471-2458-9-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S.H., Reaven G.M. Insulin resistance and hyperinsulinemia: You can’t have one without the other. Diabetes Care. 2008;31:1433–1438. doi: 10.2337/dc08-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kahn S.E. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocrinol. Metab. 2001;86:4047–4058. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 62.Satin L.S., Butler P.C., Ha J., Sherman A.S. Pulsatile insulin secretion, impaired glucose tolerance and type 2 diabetes. Mol. Asp. Med. 2015;42:61–77. doi: 10.1016/j.mam.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paolisso G., Scheen A.J., Albert A., Lefebvre P.J. Effects of pulsatile delivery of insulin and glucagon in humans. Am. J. Physiol. 1989;257:E686–E696. doi: 10.1152/ajpendo.1989.257.5.E686. [DOI] [PubMed] [Google Scholar]
- 64.O’Rahilly S., Turner R.C., Matthews D.R. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N. Engl. J. Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 65.Caumo A., Luzi L. First-phase insulin secretion: Does it exist in real life? Considerations on shape and function. Am. J. Physiol. Endocrinol. Metab. 2004;287:E371–E385. doi: 10.1152/ajpendo.00139.2003. [DOI] [PubMed] [Google Scholar]
- 66.Del Prato S., Tiengo A. The importance of first-phase insulin secretion: Implications for the therapy of type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2001;17:164–174. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- 67.Luzi L., DeFronzo R.A. Effect of loss of first-phase insulin secretion on hepatic glucose production and tissue glucose disposal in humans. Am. J. Physiol. 1989;257:E241–E246. doi: 10.1152/ajpendo.1989.257.2.E241. [DOI] [PubMed] [Google Scholar]
- 68.Basu A., Alzaid A., Dinneen S., Caumo A., Cobelli C., Rizza R.A. Effects of a change in the pattern of insulin delivery on carbohydrate tolerance in diabetic and nondiabetic humans in the presence of differing degrees of insulin resistance. J. Clin. Investig. 1996;97:2351–2361. doi: 10.1172/JCI118678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.DeFronzo R.A., Abdul-Ghani M.A. Preservation of β-cell function: The key to diabetes prevention. J. Clin. Endocrinol. Metab. 2011;96:2354–2366. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 70.Cersosimo E., Solis-Herrera C., Trautmann M.E., Malloy J., Triplitt C.L. Assessment of pancreatic β-cell function: Review of methods and clinical applications. Curr. Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zarković M., Cirić J., Penezić Z., Trbojević B., Drezgić M. Effect of weight loss on the pulsatile insulin secretion. J. Clin. Endocrinol. Metab. 2000;85:3673–3677. doi: 10.1210/jcem.85.10.6919. [DOI] [PubMed] [Google Scholar]
- 72.Escalante-Pulido M., Escalante-Herrera A., Milke-Najar M.E., Alpizar-Salazar M. Effects of weight loss on insulin secretion and in vivo insulin sensitivity in obese diabetic and non-diabetic subjects. Diabetes Nutr. Metab. 2003;16:277–283. [PubMed] [Google Scholar]
- 73.Carr D.B., Utzschneider K.M., Boyko E.J., Asberry P.J., Hull R.L., Kodama K., Callahan H.S., Matthys C.C., Leonetti D.L., Schwartz R.S., et al. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not beta-cell function. Diabetes. 2005;54:340–347. doi: 10.2337/diabetes.54.2.340. [DOI] [PubMed] [Google Scholar]
- 74.Uusitupa M., Lindi V., Louheranta A., Salopuro T., Lindström J., Tuomilehto J., Finnish Diabetes Prevention Study Group Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes. 2003;52:2532–2538. doi: 10.2337/diabetes.52.10.2532. [DOI] [PubMed] [Google Scholar]
- 75.Brown R.J., Rother K.I. Effects of beta-cell rest on beta-cell function: A review of clinical and preclinical data. Pediatr. Diabetes. 2008;9:14–22. doi: 10.1111/j.1399-5448.2007.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bodinham C.L., Smith L., Wright J., Frost G.S., Robertson M.D. Dietary fibre improves first-phase insulin secretion in overweight individuals. PLoS ONE. 2012;7:e40834. doi: 10.1371/journal.pone.0040834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kahleova H., Tura A., Hill M., Holubkov R., Barnard N.D. A Plant-Based Dietary Intervention Improves Beta-Cell Function and Insulin Resistance in Overweight Adults: A 16-Week Randomized Clinical Trial. Nutrients. 2018;10:2. doi: 10.3390/nu10020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juntunen K.S., Laaksonen D.E., Poutanen K.S., Niskanen L.K., Mykkänen H.M. High-fiber rye bread and insulin secretion and sensitivity in healthy postmenopausal women. Am. J. Clin. Nutr. 2003;77:385–391. doi: 10.1093/ajcn/77.2.385. [DOI] [PubMed] [Google Scholar]
- 79.Freeland K.R., Wilson C., Wolever T.M.S. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 80.Pingitore A., Chambers E.S., Hill T., Maldonado I.R., Liu B., Bewick G., Morrison D.J., Preston T., Wallis G.A., Tedford C., et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017;19:257–265. doi: 10.1111/dom.12811. [DOI] [PubMed] [Google Scholar]
- 81.van der Klaauw A.A., Keogh J.M., Henning E., Trowse V.M., Dhillo W.S., Ghatei M.A., Farooqi I.S. High protein intake stimulates postprandial GLP1 and PYY release. Obesity. 2013;21:1602–1607. doi: 10.1002/oby.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iverson J.F., Gannon M.C., Nuttall F.Q. Ingestion of leucine + phenylalanine with glucose produces an additive effect on serum insulin but less than additive effect on plasma glucose. J. Amino Acids. 2013;2013:964637. doi: 10.1155/2013/964637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Newsholme P., Brennan L., Rubi B., Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin Sci. 2005;108:185–194. doi: 10.1042/CS20040290. [DOI] [PubMed] [Google Scholar]
- 84.Kitabchi A.E., McDaniel K.A., Wan J.Y., Tylavsky F.A., Jacovino C.A., Sands C.W., Nyenwe E.A., Stentz F.B. Effects of high-protein versus high-carbohydrate diets on markers of β-cell function, oxidative stress, lipid peroxidation, proinflammatory cytokines, and adipokines in obese, premenopausal women without diabetes: A randomized controlled trial. Diabetes Care. 2013;36:1919–1925. doi: 10.2337/dc12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tricò D., Frascerra S., Baldi S., Mengozzi A., Nesti L., Mari A., Natali A. The insulinotropic effect of a high-protein nutrient preload is mediated by the increase of plasma amino acids in type 2 diabetes. Eur. J. Nutr. 2018 doi: 10.1007/s00394-018-1778-y. [DOI] [PubMed] [Google Scholar]
- 86.Smith G.I., Yoshino J., Kelly S.C., Reeds D.N., Okunade A., Patterson B.W., Klein S., Mittendorfer B. High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Rep. 2016;17:849–861. doi: 10.1016/j.celrep.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gower B.A., Goree L.L., Chandler-Laney P.C., Ellis A.C., Casazza K., Granger W.M. A higher-carbohydrate, lower-fat diet reduces fasting glucose concentration and improves β-cell function in individuals with impaired fasting glucose. Metabolism. 2012;61:358–365. doi: 10.1016/j.metabol.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Straznicky N.E., O’Callaghan C.J., Barrington V.E., Louis W.J. Hypotensive effect of low-fat, high-carbohydrate diet can be independent of changes in plasma insulin concentrations. Hypertension. 1999;34:580–585. doi: 10.1161/01.HYP.34.4.580. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 90.Saito T., Watanabe M., Nishida J., Izumi T., Omura M., Takagi T., Fukunaga R., Bandai Y., Tajima N., Nakamura Y., et al. Zensharen Study for Prevention of Lifestyle Diseases Group. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: A randomized controlled trial. Arch. Intern. Med. 2011;171:1352–1360. doi: 10.1001/archinternmed.2011.275. [DOI] [PubMed] [Google Scholar]
- 91.Weber M.B., Ranjani H., Staimez L.R., Anjana R.M., Ali M.K., Narayan K.M., Mohan V. The Stepwise Approach to Diabetes Prevention: Results From the D-CLIP Randomized Controlled Trial. Diabetes Care. 2016;39:1760–1767. doi: 10.2337/dc16-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thankappan K.R., Sathish T., Tapp R.J., Shaw J.E., Lotfaliany M., Wolfe R., Absetz P., Mathews E., Aziz Z., Williams E.D., et al. A peer-support lifestyle intervention for preventing type 2 diabetes in India: A cluster-randomized controlled trial of the Kerala Diabetes Prevention Program. PLoS Med. 2018;15:e1002575. doi: 10.1371/journal.pmed.1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rautio N., Jokelainen J., Oksa H., Saaristo T., Peltonen M., Puolijoki H., Tuomilehto J., Vanhala M., Moilanen L., Uusitupa M., et al. Changes in glucose metabolism in people with different glucose metabolism disorders at baseline: Follow-up results of a Finnish national diabetes prevention programme. Diabet. Med. 2015;32:1611–1616. doi: 10.1111/dme.12776. [DOI] [PubMed] [Google Scholar]
- 94.Unwin N., Shaw J., Zimmet P., Alberti K.G. Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet. Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 95.Færch K., Witte D.R., Tabák A.G., Perreault L., Herder C., Brunner E.J., Kivimäki M., Vistisen D. Trajectories of cardiometabolic risk factors before diagnosis of three subtypes of type 2 diabetes: A post-hoc analysis of the longitudinal Whitehall II cohort study. Lancet Diabetes Endocrinol. 2013;1:43–51. doi: 10.1016/S2213-8587(13)70008-1. [DOI] [PubMed] [Google Scholar]
- 96.Faerch K., Borch-Johnsen K., Holst J.J., Vaag A. Pathophysiology and aetiology of impaired fasting glycaemia and impaired glucose tolerance: Does it matter for prevention and treatment of type 2 diabetes? Diabetologia. 2009;52:1714–1723. doi: 10.1007/s00125-009-1443-3. [DOI] [PubMed] [Google Scholar]