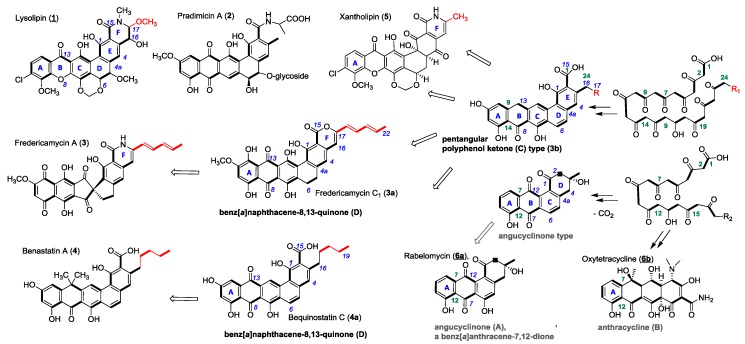
Figure 1.
Chemical structures of PKS II products (1–6) and examples of biosynthetic congeners (3a, b, 4a). PKS chains (acetate units bold) of angucyclinone (A) and anthracycline (B) type structures start to cyclize with ring A at C-7/C-12. Benz[a]naphthacene (C) and benz[a]naphthacene quinone (D) type structures cyclize starting with C-9/C-14 according to polyketide chain numbering (green). Variations of δ-substituents of ring-F highlighted in red. R = H or alkyl-, allylic carbon chains (usual chemical nomenclature in blue).