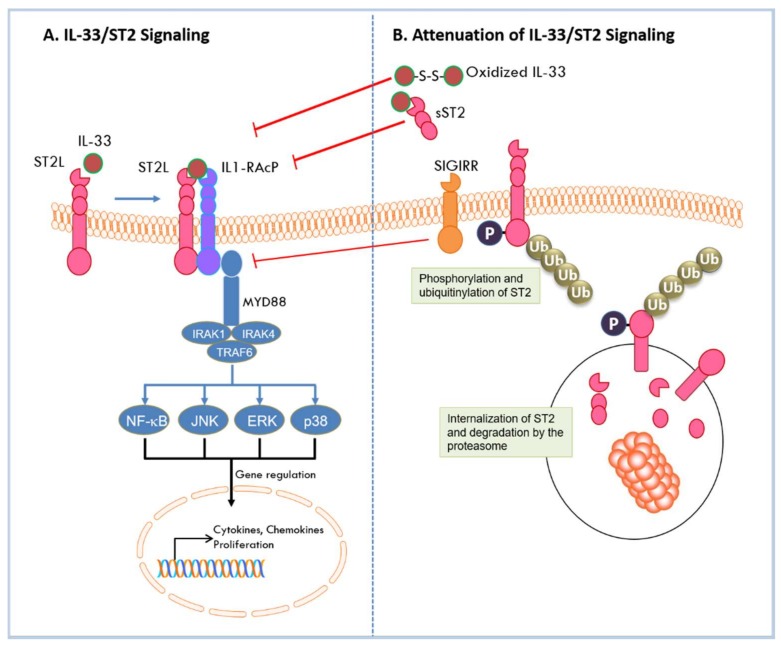
Figure 2.
Activation and Attenuation of IL-33/ST2 Signaling. (A) IL-33/ST2 Signaling. IL-33 binds to ST2L causing a conformational change that leads to recruitment of IL1-RAcP. Heterodimerization of the transmembrane proteins results in interaction between their intracellular C-terminal domains that facilitates recruitment of adaptor molecules including MyD88, IRAK1, IRAK4, and TRAF6. Subsequent activation of transcription factors NF-κB, JNK, ERK, and p38 leads to expression of genes encoding cytokines, chemokines, and growth factors. (B) Attenuation of IL33-ST2 signaling. SIGIRR can disrupt the ST2L/IL1RAcP heterodimer. Phosphorylated ST2L is quickly internalized, polyubiquitinylated by the E3 ligase FBXL19 and targeted for degradation by the proteasome. Extracellular IL-33 can be sequestered by sST2 acting as a molecular decoy and can be quickly oxidized via its cysteine residues. Both processes prevent its interaction with the membrane bound ST2L receptor. Blue arrows indicate interaction between molecules, black arrows indicate gene regulation or transcriptional activation; red lines with bars indicate disruption of molecular interactions.