Figure 5.
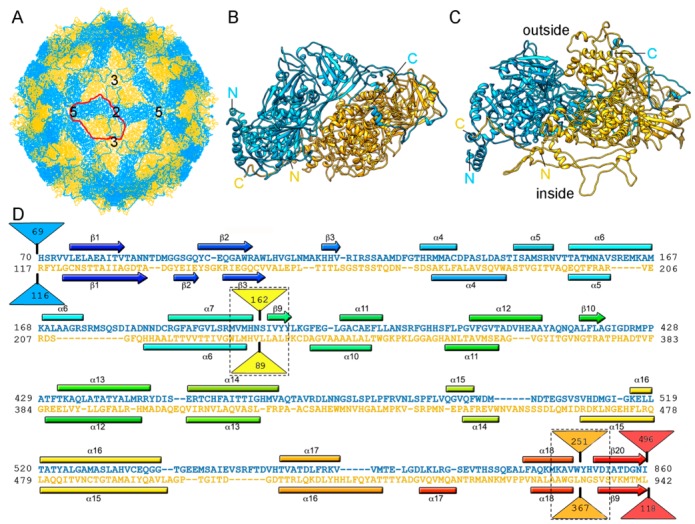
RnQV1 T = 1 capsid cryo-EM-based structure. (A) T = 1 capsid of RnQV1 viewed along a two-fold axis of icosahedral symmetry, showing P2 (blue) and P4 (yellow). Boundaries for an asymmetric unit are outlined in red. Numbers indicate icosahedral symmetry axes. (B) Top and (C) side views of the atomic models of P2 (blue; 972 residues) and P4 (yellow; 1005 residues) (5nd1). The last visible P2 C-terminal residue is located on a P4 surface crevice. (D) Sequence alignment of P2 (blue) and P4 (yellow) resulting from Dali structural alignment. α-helices (rectangles) and β-strands (arrows) are rainbow-colored from blue (N terminus) to red (C terminus) for each protein. Dashed rectangles indicate favorable insertion sites, triangles represent non-aligned segments (sizes indicated).
