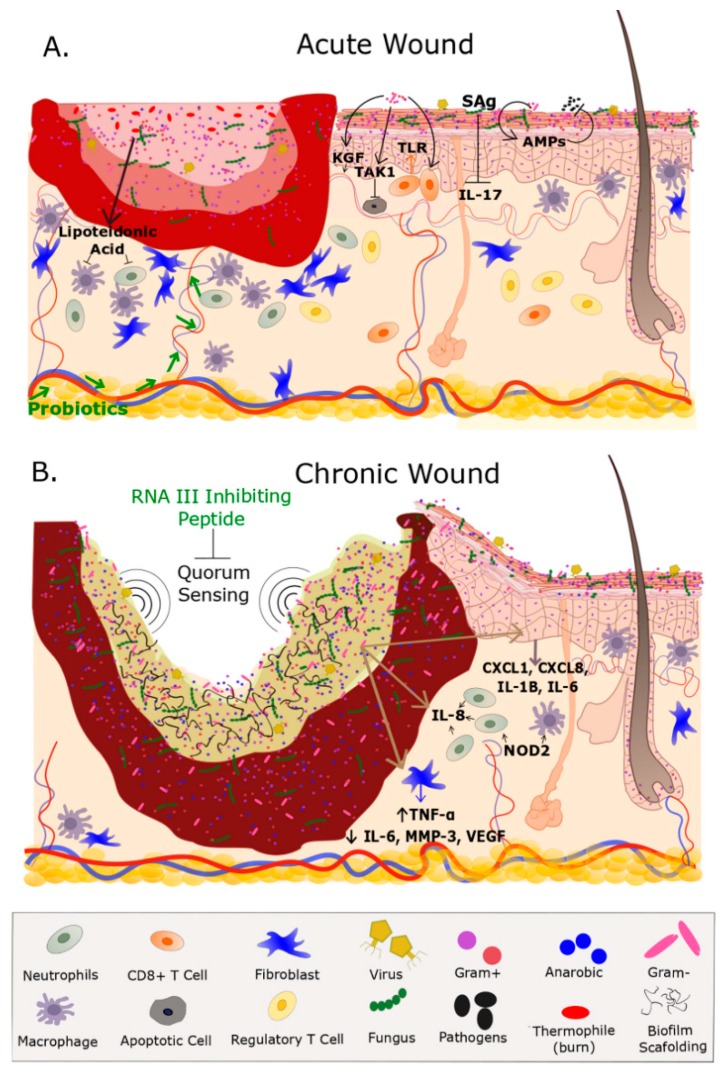
Figure 1.
Microbiome, Wound Healing, and Wound Healing Impairment. (A) Acute wounds such as burns and open fractures contain a microbiome that may or not contain a vastly different population than what is on undamaged adjacent skin. The microbes present on an acute wound don’t necessarily impede the inflammatory response therefore allowing for the highly coordinated events that promote wound healing. Several fibroblast, and inflammatory cells such as macrophages, neutrophils, and T Cells are highly involved in the healing process. Production of lipoteichoic acid by S. epidermidis deceases inflammation. Keratinocytes express anti-microbial peptides (AMPs) in response to S. epidermidis, S. aureus, Group A streptococcus which provides protection for pathogenic bacterium. Supplementation with probiotics (e.g., Lactobacillus reuteri) accelerates wound healing although the mechanism is unknown. CD8+ T Cells in response to S. epidermidis enhance rapid keratinocyte progression via Toll-like receptor (TLR). Additionally, Pseudomonas accelerates epithelization and blood vessel growth through transforming growth factor beta-activated kinase 1 (TAK1) signaling. Overabundance of S. aureus produces superantigens (Sag) that are decreases interleukin (IL-17) and subsequently promoting wound healing. Note the greater numbers of healthy fibroblast, macrophages, and neutrophils near the wound bed and the intact vasculature that are essential in the healing process. Adjacent to the wound is undamaged (healthy) skin that contains macrophages, fibroblast, and a healthy ecological community of microorganisms that includes Gram Negatives, Gram Positives, fungi, and viruses within the epidermis and hair shaft; (B) chronic wounds contain a biofilm and a dense population of microorganisms which include anaerobic bacteria that obstruct wound healing by preventing topical antibiotics reaching the wound bed. Quorum sensing within the biofilm promotes biofilm formation, whereas exogenous topicals such as RNA inhibiting peptide inhibits biofilm formation. The biofilm elevates the expression of cytokines IL-1B, IL-6, chemokine ligand (CXCL) 1 and 8. Bacterium in the biofilm increase levels of IL-8 which is a potent neutrophil chemoattractant. Additionally, the biofilm increases levels of TNF-α, and decreases IL-6, MMP-3, and vascular endothelial growth factor (VEGF). Nucleotide-binding oligomerization domain-containing protein 2 (NOD2) stimulates a host response and is highly expressed in chronic wounds. The loss of vasculature to the wound bed further prevents the migration of immune-related factors and delivery of exogenous therapeutics. Fibroblast migration is impeded by biofilm formation Note the hyper-proliferative epidermis on the outer region of the wound bed in an attempt to epithelialize the wound. Although inflammation is present in chronic wounds the overall number of normal functioning fibroblasts and macrophages are low which further prevents healing. Green font represents an exogenous treatment for wound healing.