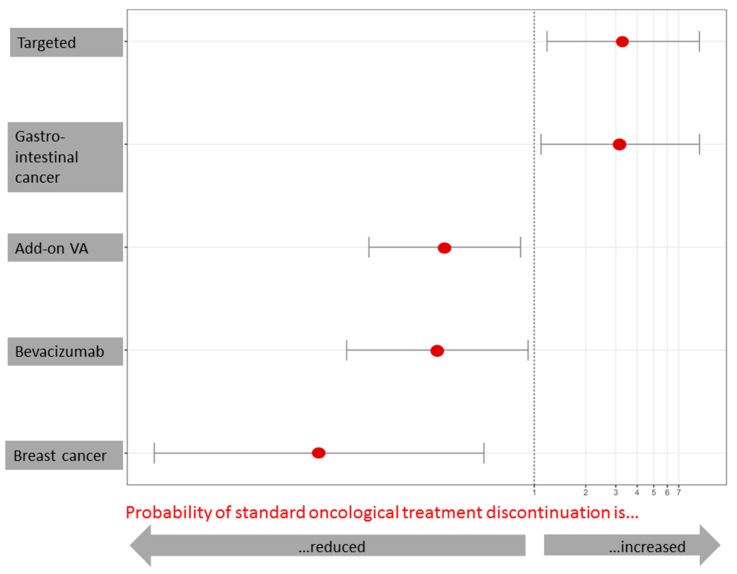
Figure 4.
Probability of oncological treatment discontinuation in the AE-experiencing subgroup (n = 34). Adjusted multivariable logistic regression analysis. Factors presented are statistically significant (p < 0.05) for reduced probability (left-hand side from the indicated margin) or for increased probability (right -hand side from the indicated margin) of oncological treatment discontinuation due to adverse side effects, i.e., three times higher probability of discontinuation of standard oncological therapy when applying targeted compared to combined therapy, three times higher probability of discontinuation of standard oncological therapy in gastrointestinal tumors compared to non-gastrointestinal tumors, 70% reduced risk of discontinuation of standard oncological therapy when applying add-on VA therapy compared to targeted therapy without add-on VA therapy, 73% reduced risk of discontinuation of standard oncological therapy when applying bevacizumab compared to therapy without bevacizumab, 94% reduced risk of discontinuation of standard oncological therapy in breast cancer compared to non-breast cancer.