Table 1.
Summary of clinical evidence of dronabinol, nabilone, and nabiximols.
| Dronabinol (Synthetic∆9-THC) | Nabilone (Synthetic Analogue of ∆9-THC) | Nabiximols (∆9-THC: Cannabidiol~1:1 (w/w)) | |
|---|---|---|---|
| Structure(s) |
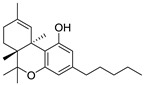
|
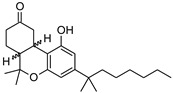
|
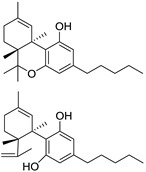
|
| Formulation | Soft gelatin capsules (2.5, 5, 10 mg) | Capsules (0.25, 0.5, 1 mg) | Oro-mucosal spray (27 mg of ∆9-THC and 25 mg of cannabidiol/1.0 mL) |
| Disability and disease progression | No evident changes | No studies | No evident changes |
| Pain | Positive effects | Positive effects | Mixed findings (mostly positive effects) |
| Spasticity | Mixed findings | Positive effects | Mixed findings (mostly positive effects) |
| Bladder function | Mixed findings | Positive effects | Mixed findings |
| Ataxia and tremor | No evident changes | No studies | No evident changes |
| Sleep | Mixed findings (mostly positive effects) | No studies | Positive effects |
| Quality of life | Mixed findings | Mixed findings (moslty positive effects) | Mixed findings |
| Adverse effects | Mild to moderate. Principally dizziness, euphoria, dry mouth, fatigue and drowsiness. | Moderate sedation, dizziness and moderate weakness in the legs. | Mild to moderate. Principally drowsiness, dizziness, headache, fatigue, impaired balance and disturbance in attention. |
| Number of studies | 10 | 3 | 11 |
| Number of reviews | 11 | 5 | 12 |
| Studies (references) | [45,46,47,48,49,50,51,52,53,54] | [55,56,57] | [58,59,60,61,62,63,64,65,66,67,68] |
| Reviews (references) | [33,69,70,71,72,73,74,75,76,77,78] | [69,70,72,73,78] | [69,70,72,73,74,75,76,77,78,79,80,81] |
