Figure 3.
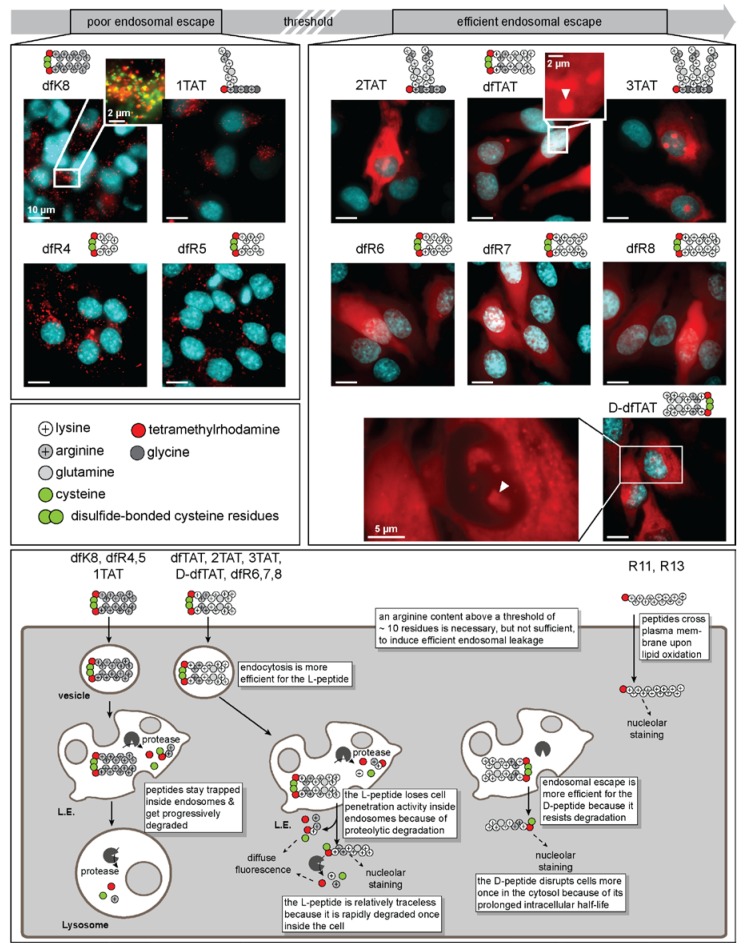
Active vs. inactive dfTAT variants. A threshold guanidinium density must be reached to achieve successful cytosolic penetration. Peptides such as dfK8, 1TAT, dfR4, and dfR5 do not meet the threshold guanidinium density, and as such, remained trapped within endosomes, as seen as puncta in the included fluorescence microscopy images. While trafficking through the endocytic pathway, these peptides become progressively more degraded. However, once a peptide has met or exceeded the guanidinium threshold, successful cytosolic penetration is obtainable (nucleolar staining is used to confirm that the fluorescence signal is intracellular and is highlighted with white arrows). This is shown as fluorescence microscopy images over 2TAT, dfTAT, 3TAT, dfR6, dfR7, and dfR8. Owing to susceptibility to proteolysis, l-peptides are degraded along the endocytic pathway. This results in a decreased activity upon reaching the late endosome. However, one favorable outcome of this characteristic is that upon successful cytosolic penetration, l-peptides are relatively traceless, as they are also rapidly degraded within the cell. Notably, peptides that are protease resistant (e.g., d-amino acid peptides) are less efficient at inducing endocytosis, but more efficient at causing endosomal escape relative to l-amino acid counterparts. These peptides remain intact and localize to the nucleoli of the cell for a prolonged period of time (>3 days; in contrast, l-peptides are fully degraded within 3 h, leading to a disappearance of nucleolar staining). As a drawback, d-peptides disrupt cells more once in the cytosolic space, owing to the prolonged intracellular half-life. Finally, linear, non-branched peptides that have a high guanidinium content (e.g., R11, R13) enter cells by direct plasma membrane translocation. In our hands, this process is dependent on culture conditions, membrane oxidation, and lipid peroxidation. Why branched and linear species differ in their internalization routes remains unclear. This nonetheless suggests that arginine content alone is not sufficient to confer endosomolytic activity, and that other chemical or structural features must be at play in compounds such as dfTAT.