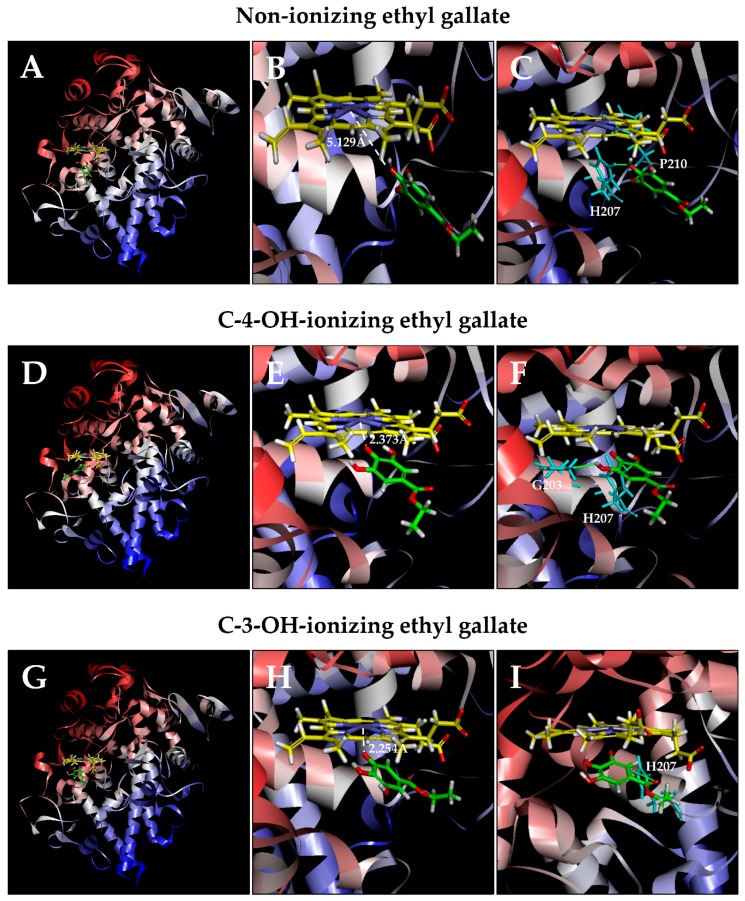
Figure 5.
Molecular docking analysis of the binding interaction of ethyl gallate with the peroxidase active site of the COX-1 protein. Panels A, D, and G: The dominant docking result for non-ionizing (A), C-4-OH-ionizing (D), and C-3-OH-ionizing (G) ethyl gallate inside the peroxidase active site of COX-1. The protein structure is shown in a flat ribbon format. In P+FeIV, carbon is colored in yellow, nitrogen in blue, oxygen in red, hydrogen in white, and iron in bice. In ethyl gallate, carbon is colored in green, oxygen in red, and hydrogen in white. Panels B, E, and H: The same structures as in panels A, D, and G with a white dashed line added to indicate the distance between Fe4+ ion and O of one of the OH groups in ethyl gallate. Panels C, F, and I: Suggested potential hydrogen bonds (green lines) between the non-ionizing (C), C-4-OH-ionizing (F), or C-3-OH-ionizing (I) ethyl gallate and the amino acid residues (Gln203, His207, Phe210) in the peroxidase site. The amino acid residues are colored in light blue.