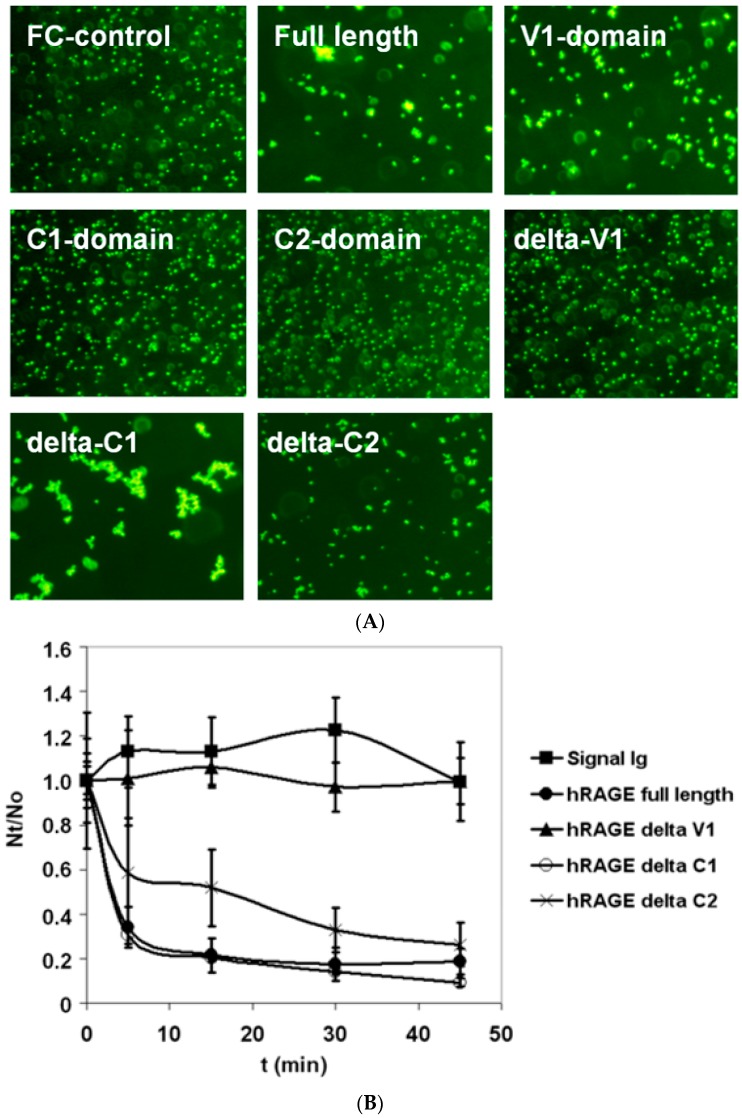
Figure 1.
Bead aggregation assay of the receptor for advanced glycation end products (RAGE) homophilic binding. (A) Monomeric fluorescent protein A-coated beads were incubated with low concentration (10 μg/mL) of different Ig-tagged recombinant full length extracellular human RAGE (sRAGE) domains and aggregation was monitored using fluorescence microscopy. The images are taken after 60 min of bead aggregation. (B) Kinetics of bead aggregation. Nt and N0 are the total number of particles at incubation times t and 0, respectively. The extent of bead aggregation is represented by the index Nt/N0. Closed circle curve represents full-length sRAGE–Ig fusion protein, close triangle curve represents sRAGE–Ig fusion protein lacking V1-domain, open circle curve represents sRAGE–Ig fusion protein lacking C1-domain, cross curve represents sRAGE–Ig fusion protein lacking C2-domain, and close square curve represents Fc control protein coated beads. Whereas V1-domain was necessary and sufficient for homophilic binding of RAGE, the existence of the C2-domain in recombinant sRAGE-fragment was required for maximal homophilic binding of RAGE forms bearing the V1- and C2-domains. N ≥ 3; error bars represent ±SD.