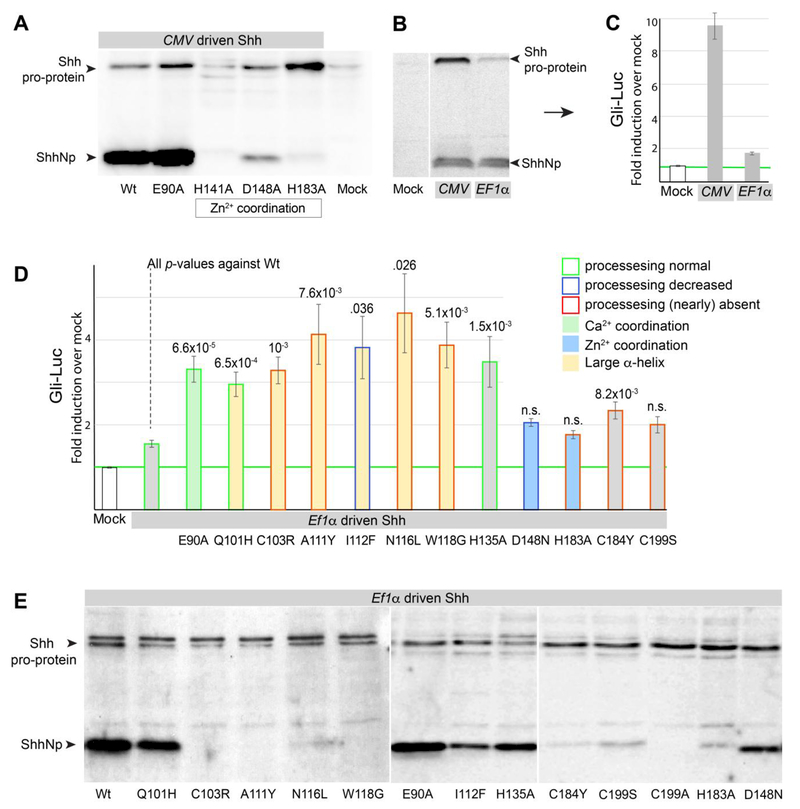
Figure 5. Shh processing is not required for the cell-autonomous activation of the Hh response in Ptch1LacZ/LacZ;Ptch2−/− cells.
(A) Shh western blot showing that mutations in the Zn2+ coordination triad (H141A, D148A, H183A) severely compromise, or prevent Shh processing. E90A (affecting Ca2+ coordination) undergoes normal processing. (B) Shh western blot showing expression levels (high for the CMV and low for the EF1α promoter) affect the level of Shh pro-protein more so than ShhNp. (C) The induction of the Gli-Luciferase was measured after transfection of EF1α- or CMV-driven Shh. The induction of the response correlates with the amount of Shh pro-protein, not ShhNp. (D) Gli-Luciferase induction by Shh mutants driven off the EF1α promoter. Several HPE mutants located in the large α-helix (Q101H, C103R, A111Y, I112F, N116L, W118G), mutants involved in Zn2+ coordination (D148N, H183A), E90A (Ca2+ coordination), H135 (near the catalytic domain), C184Y (commonly mutated in HPE), and C199S (cleavage site for auto-processing, mutated in HPE) were assessed. Error bars for E are s.e.m., p values (Student t-test, 2 tailed) are indicated were relevant, n=3 of independent biological replicates of triple or quadruple independent parallel experiments. (E) Western blot analysis of EF1α-driven Shh mutants assessed in F. Note that the ability to auto-process is often impaired in α-helix and Zn2+ coordination mutants.