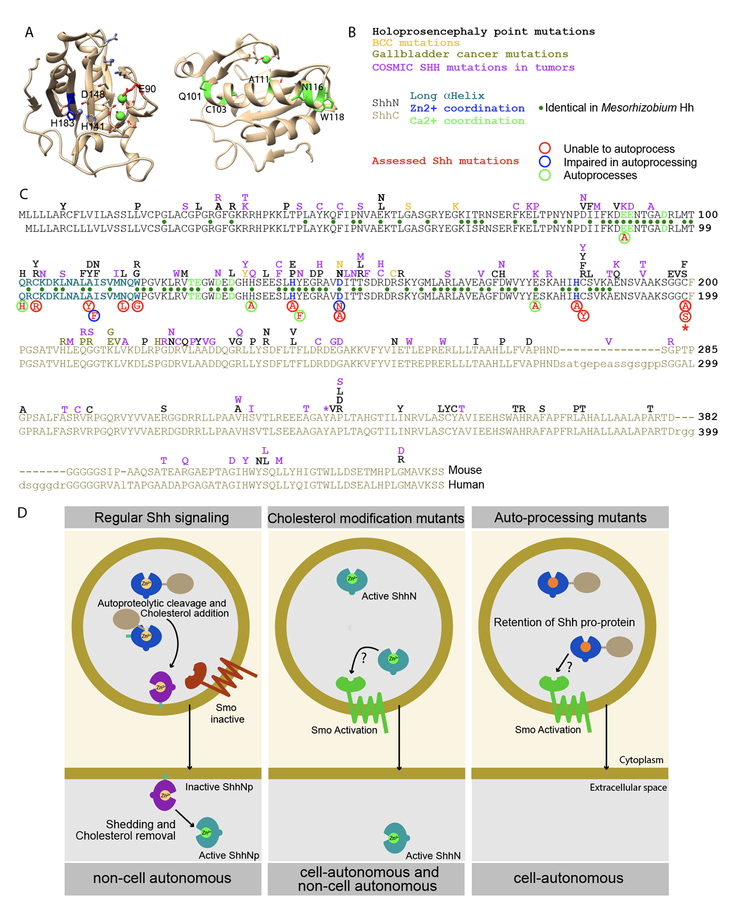
Figure 6. Overview and model.
Representation of the ShhN crystal structure with salient residues indicated. On the left is a view into the Zn2+ (grey)/Ca2+ (green) coordination domains, on the right is the opposite view with the large α-helix in front, with mutated residues indicated in green. (B, C) Lineup of the mouse (top) and human Shh. All tested mutants are indicated below the lineup, with their activity and ability to process indicated. Above the line are Shh point mutations described for holoprosencephaly, BCCs, Gallbladder Cancers, and those curated in the COSMIC database. Identical residues found in Mesorhizobium Hh are indicated with green dots between the lines. (D) During regular Hh signaling, the potentially active pro-protein auto-cleaves to yield the inactive ShhNp form. Further processing mediates activation and release, thus allowing signaling in trans. Mutants that do not receive the cholesterol modification always exist in an active form, thus mediating both cell-autonomous and non-cell autonomous signaling. Repressed auto-processing of the Shh pro-protein results in perdurance of this active form, thus causing cell-autonomous activation of the Hh response.