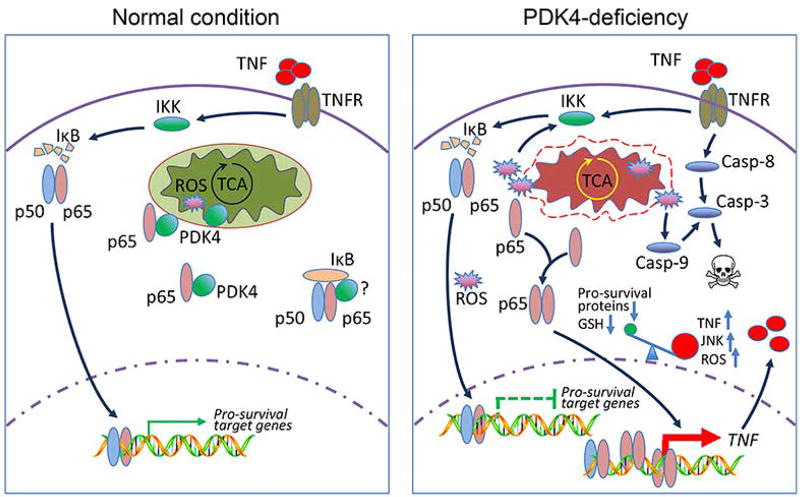
Figure 8.
Schematic showing PDK4 as a checkpoint in NF-κB/TNF-mediated apoptosis. Under normal cellular conditions, PDK4 interacts with NF-κB/p65 and sequesters p65 in the cytoplasm. Mitochondria respiration and ROS production remain normal. TNF treatment can activate NF-κB (p50/p65 complex) signaling to promote the induction of pro-survival genes. Knockdown of PDK4 disrupts mitochondria respiration and generates excessive ROS; the latter facilitates PDK4 and p65 protein dissociation, and releases p65 to accelerate its nuclear translocation. Excessive ROS may also deplete cellular GSH and activate JNK. In this scenario, in the nucleus of PDK4-deficient cells, pro-survival genes are less/not responsive to p65 transactivation, whereas TNF is activated which in turn induces caspase-8 and the extrinsic apoptosis pathway. In addition, ROS leads to activation of caspase-9 and the intrinsic apoptosis pathway. Collectively, we propose that increased ROS, decreased GSH, and sustained JNK activation shift the pro-survival function NF-κB toward facilitating TNF-mediated apoptosis.