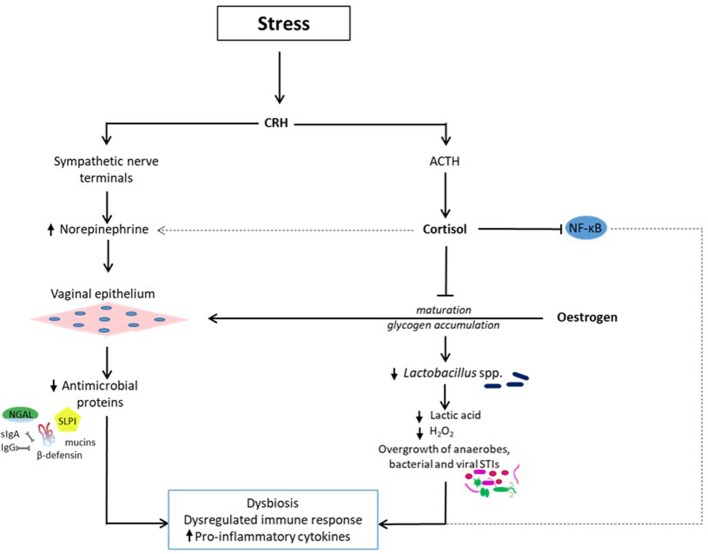
Figure 1.
Stress-related reduction in vaginal Lactobacillus dominance (dysbiosis) and dysregulated immune response. Exposure to psychosocial stress induces the release of cortisol and norepinephrine via the hypothalamic-pituitary-adrenal and SAM axes respectively. Cortisol inhibits the estrogen-related vaginal epithelial maturation and glycogen accumulation. Levels of vaginal free glycogen and Lactobacilli are reduced leading to decreased lactic acid and hydrogen peroxide (H2O2) synthesis and pH. Consequently, a dysbiotic environment conducive for the proliferation of bacterial vaginosis-associated anaerobic bacteria such as Gardnerella, Prevotella, Mobiluncus, Atopobium, Megasphera, and sexually transmitted infections such Neisseria gonorrhea, Chlamydia trachomatis, human immunodeficiency virus is created. Cortisol also affects immune response by altering the nuclear factor-κB (NF-κB) signal transduction pathway, which regulates inflammatory gene expression. These effects are exacerbated by the concurrent release of norepinephrine, which binds to vaginal epithelial cells and potentiates the pro-inflammatory response via a reduction in the release of antimicrobial proteins including mucins, immunoglobulins (secretory Ig A and IgG), β-defensins, secretory leucocyte protease inhibitor (SLPI), and neutrophil gelatinase-associated lipocalin (NGAL). The overall effect is a dysbiotic vaginal ecosystem with a sub-optimal immune response, which encourages upper genital tract infection with deleterious gynecological and obstetric sequelae.