Figure 5. The histone‐binding motif in the amino‐terminal tail of the DNA polymerase subunit of Pol α is specific for histones H2A‐H2B.
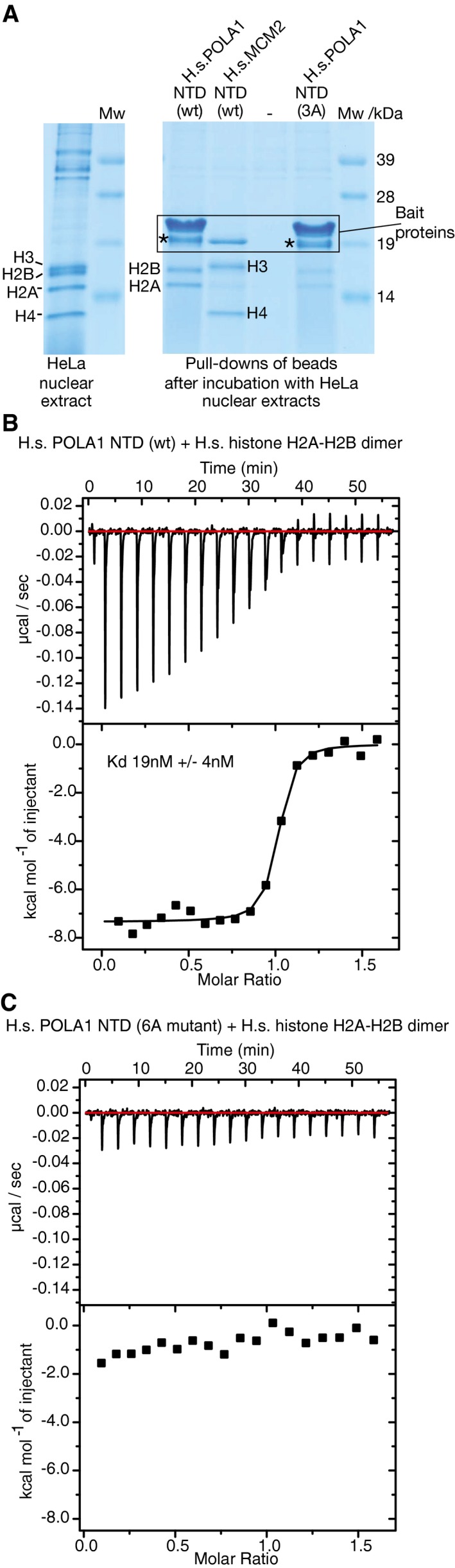
- Recombinant versions of the amino‐terminal tail of human POLA1 (amino acids 1–110 of the polymerase subunit of Pol α) or human MCM2 (residues 43–160) were purified from Escherichia coli as fusions to a Twin‐Strep‐tag. The Strep‐Tactin beads carrying the purified tails were then incubated with a high‐salt extract of HeLa nuclei, before the beads were washed and the bound proteins eluted in d‐Desthiobiotin buffer. The eluted proteins were revealed by SDS–PAGE and Coomassie staining, and relevant bands were identified by mass spectrometry and confirmed by immunoblotting. The asterisk denotes a degradation product of the POLA1‐NTD.
- The interaction of recombinant H.s. POLA1‐NTD with a complex of recombinant histones H2A‐H2B was monitored by isothermal titration calorimetry. The experiment was performed three times, and the figure shows a representative example.
- Similar analysis of the interaction of H.s. POLA1‐NTD (6A mutant) with a complex of recombinant human histones H2A‐H2B.
