Figure 3.
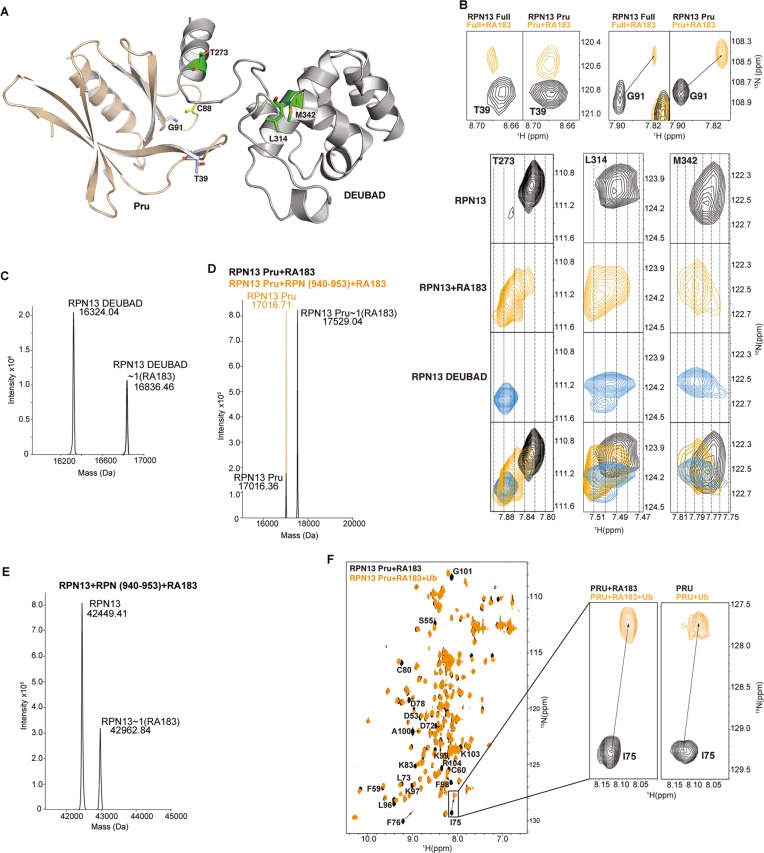
RA183 impacts RPN13 interdomain interactions, but not binding to ubiquitin. (A) Full-length RPN13 structure with amino acids highlighted in (B) displayed and labeled. The RPN13 Pru and DEUBAD domains are colored wheat and gray, respectively, with T39 and G91 in purple; T273, L314, and M342 in green; and C88 in yellow; the side-chain nitrogen, oxygen, and sulfur atoms of these amino acids are in blue, red, and yellow, respectively. (B, top) Expanded regions highlighting T39 and G91 of the RPN13 Pru domain from (1H, 15N) HSQC spectra recorded on 15N-labeled RPN13 (black, left) and RPN13 Pru domain (black, right) and after incubation with 10-fold molar excess RA183, followed by dialysis for removal of excess and labile RA183 (orange). (B, bottom) Expanded regions highlighting T273, L314, and M342 from (1H, 15N) HSQC spectra recorded on 15N-labeled RPN13 (black) and after incubation with 10-fold molar excess RA183, followed by dialysis for removal of excess and labile RA183 (orange), and RPN13 DEUBAD domain (third row). The top two panels are derived from the same spectra as used to generate those for T39 and G91. A merger of the top three rows is displayed in the bottom row with RPN13, RPN13 with RA183, and RPN13 DEUBAD in black, orange, and blue, respectively. (C–E) LC–MS analysis of 2 μM RPN13 DEUBAD (C, left), Pru without (D, black) or with equimolar RPN2 (940–953) (D, orange), or full-length RPN13 (E) following 2 h of incubation with 20 μM RA183. (C, right) Expanded regions highlighting RPN13 Pru domain C88 (boxed inset) and RPN13 DEUBAD domain L311, C357, Q358, and F359 from (1H, 15N) HSQC spectra recorded on 15N-labeled RPN13 (black) and after incubation with 10-fold molar excess RA183, followed by buffer exchange to remove excess and labile RA183 (orange). (F) (1H, 15N) HSQC spectra of 15N-labeled RPN13 Pru domain after incubating with 10-fold molar excess of RA183, followed by dialysis to remove excess and labile RA183 (black) and with equimolar ubiquitin (orange). Resonances effected significantly are labeled, and a comparison to unligated RPN13 (black) with ubiquitin (orange) is provided for I75, which is at the ubiquitin-binding surface.