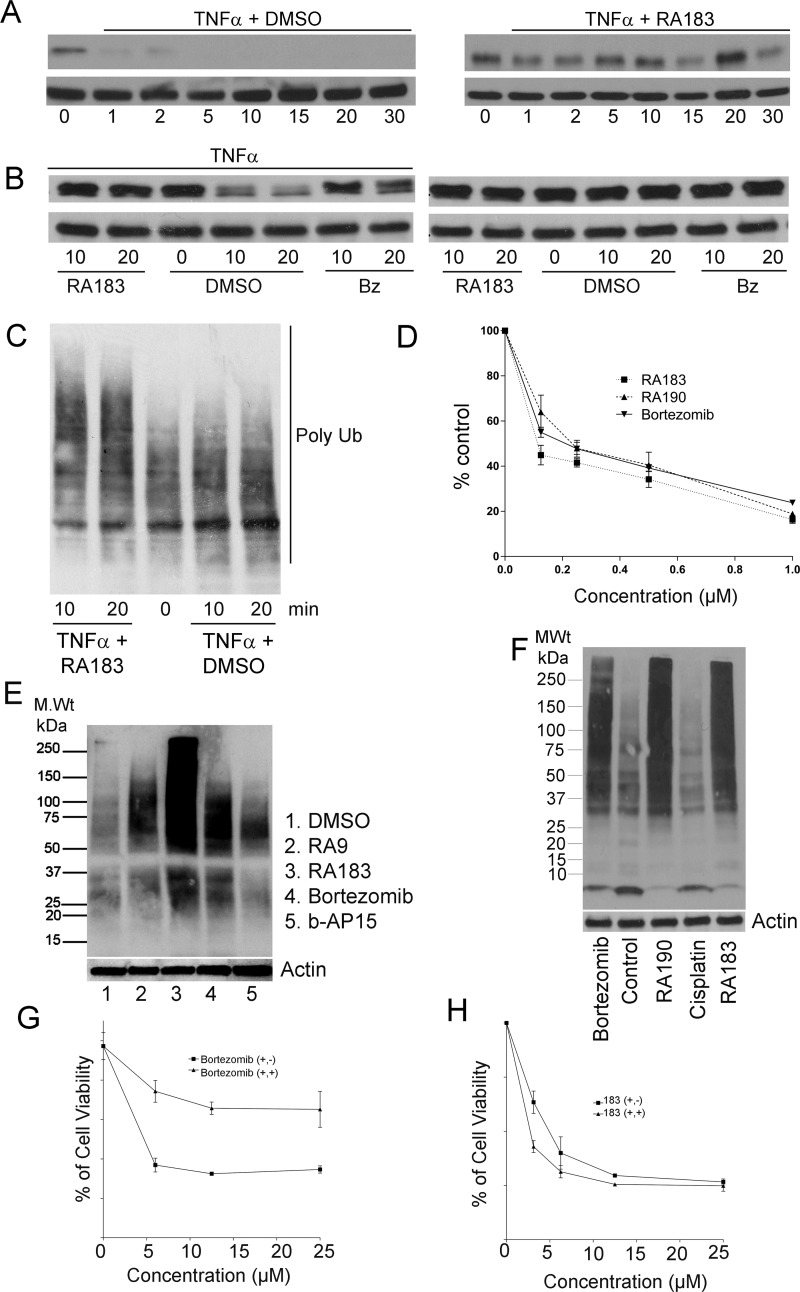
Figure 4.
RA183 blocks NFκB signaling and irreversibly induces cell death. (A) HeLa cells were simultaneously treated with 10 ng/mL TNFα and 1 μM RA183 (right) or 10 ng/mL TNFα only as a control (left). Whole cell lysates were prepared at the indicated times and analyzed by Western blot for IκB and β-tubulin levels. (B) HeLa cells were treated with 10 ng/mL TNFα (left) or not (right) and 1 μM RA183 or bortezomib (Bz) or dimethyl sulfoxide (DMSO) only as a control. Whole cell lysates were harvested 10 or 20 min later and analyzed by Western blot for IκB and β-tubulin levels. (C) Same as in (A), but probed with antibody to Lys48-coupled ubiquitin. (D) Two hundred ninety-three cells transiently transfected 1 day earlier with a luciferase reporter construct driven by either an NFκB-dependent promotor (NFκB-Luc) or a constitutive promoter (Luc) were treated with the compounds indicated and TNFα (10 ng/mL) for 7 h. Upon the addition of luciferin, bioluminescence was measured in cell lysates using a luminometer. The % activation of the NFκB-dependent promoter (normalized by the constitutive reporter construct) as compared to TNFα stimulation in the absence of compound is presented. (E) SKOV3 cells were treated with compounds (0.5 μM) for 4 h, lysed, and subjected to Western blot analysis with Ub antibody. (F) SKOV3 cells were treated with bortezomib, RA190, cisplatin, or RA183 (1 μM) for 12 h. Cell lysates were analyzed by Western blot analysis with antibody to ubiquitin. (G, H) The viability of HeLa cells treated with bortezomib (G) or RA183 (H) or at the indicated concentrations for 24 h (+, +) was compared to 1 h treatment with the same compounds at the same concentrations, after which the cells were washed and the medium was replaced without compounds and incubated for a further 23 h (+, −). Cell viability was measured by the XTT method.