Short abstract
Objective
This study was performed to discuss the characteristics, diagnosis, and treatment of primary prostatic extragastrointestinal stromal tumor (EGIST).
Methods
The case history data of a patient with an EGIST were analyzed and discussed with a literature review.
Results
The patient was diagnosed with a pelvic tumor, possibly malignant. We ascertained the diagnosis by exploratory surgery and pathological biopsy. The tumor was present in the prostate and infiltrated and pressed against the anterior rectal wall. Pathological biopsy showed that the tumor comprised spindle cells, which were also present at the junction of the tumor and prostate tissue. Immunohistochemically, the tumor cells were positive for CD117, DOG-1, CD34, and smooth muscle actin and negative for S100 and desmin; Ki-67LI was about 10%. These results support the diagnosis of primary prostatic EGIST.
Conclusion
The rarity and nonspecific clinical manifestation of prostatic EGIST facilitate misdiagnosis. Diagnosis mainly depends on imaging examination and characteristic histopathological and immunohistochemical features, and GIST must be excluded. Surgery is the main treatment method, and imatinib is suggested for unresectable and malignant EGISTs.
Keywords: Extragastrointestinal stromal tumor, prostate, clinical manifestation, diagnosis, treatment, imatinib
Introduction
As a tumor with low incidence, gastrointestinal stromal tumor (GIST) was named owing to its origination from gastrointestinal mesenchymal tissue, which mainly consists of spindle cells, epithelioid cells, and polymorphic cells. CD117 (c-kit receptor) and CD34 are important biomarkers of GIST. Extragastrointestinal stromal tumors (EGISTs) are a group of soft tissue tumors that originate from outside the gastrointestinal tract and have pathological characteristics, immunohistochemical biomarkers, and molecular biological characteristics similar to those of GISTs. EGIST is frequently reported in the omentum, abdominal cavity, and retroperitoneal soft tissue, but it has rarely been reported in the prostate. A patient with a primary prostatic EGIST was admitted to the Third Affiliated Hospital of Jianghan University in November 2014. We herein present the detailed information of this case. This rare case is being reported to discuss the clinical presentation, differential diagnosis, pathologic characteristics, and therapeutic strategies for primary prostatic EGIST.
Case report
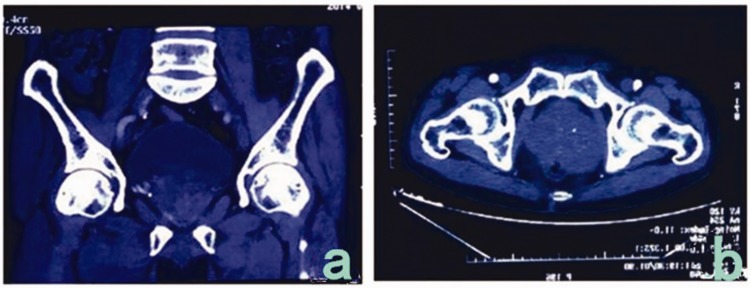
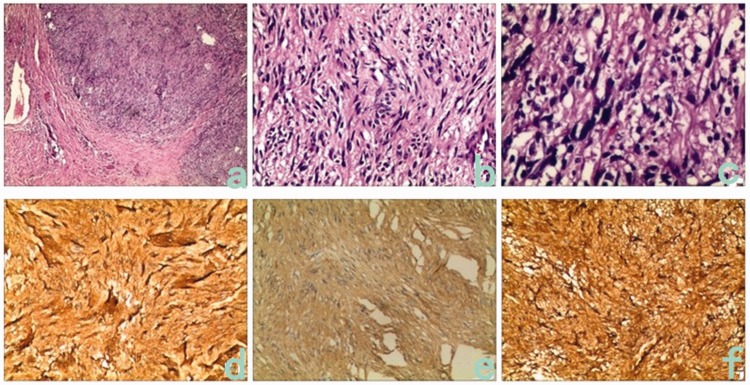
A 66-year-old man was admitted to our hospital in November 2014 because of a >2-month history of an intermittent defecation abnormity. The patient’s condition was complicated by tenesmus, feelings of incomplete defecation with a defecation interval of 3 to 4 days, and dry stool. His abdomen was flat, and no gastric peristaltic wave or varicosity of the abdominal wall was observed. No swelling of the liver or spleen was present. Pressure-induced tenderness was present over the lower abdomen, but no obvious mass was palpated and no shifting dullness or succussion splash was heard. No percussion tenderness was present over the hepatic and kidney regions. Additionally, no obvious increase or decrease in borborygmus was observed. Digital rectal examination revealed a solid mass in the anterior rectal wall. The mass had an irregular shape and was sessile; it was difficult to determine the size of the mass. It was located 5 cm away from the anus, and no blood was found upon digital palpation. Plain computed tomography (CT) of the pelvis was performed (Figure 1(a), (b)) and revealed asymmetrical enlargement of the prostate (7.6 × 7.6 × 8.7 cm), even enhancement density, a clear fat space in the prostatic surroundings, an obscure right seminal vesicle, and no abnormal thickening in the bladder wall. No obvious lymph node enlargement or hydrops was present in the pelvis. No abnormality was observed in the pelvic wall structure or pelvic floor structure. Magnetic resonance imaging (MRI) showed an enlarged prostate (7.5 × 7.5 × 8.5 cm) with an abnormal shape, abnormal radiofrequency signals, and ill-defined lesions with unclear margins. The enlarged prostate pressed backward on the rectum, but no significant abnormality was found during bone scanning. The total prostate-specific antigen (PSA) level was 2 ng/mL, the complex PSA level was 1.61 ng/mL, and the ratio of the complex to total PSA levels was 0.79. Colonoscopy showed a 4.0- × 4.0-cm protrusion 5 cm away from the anus, with superficial erosion/ulceration, congestion, and swelling of the surrounding mucous membrane, a narrow lumen, and difficulty inserting the colonoscope. We concluded that the protrusion in the rectum had possible resulted from pressure outside the lumen. No prostate biopsy was conducted because of the lack of facility in the hospital. Exploratory laparotomy was performed because of the patient’s clinical manifestations and surgical indications. The preoperative diagnosis was a pelvic tumor, possibly malignant. A tumor of approximately 8.0 × 7.0 × 7.0 cm was observed during the operation; it originated from the prostate and had invaded and pressed against the anterior rectal wall. Therefore, radical prostatectomy + rectum repair + sigmoid colostomy was performed. The postoperative histopathologic report (Figure 2(a)–(c)) described a tumor with a maximum diameter of 7 cm, infiltration of diffused spindle cells, slight pathological changes of the cell nuclei, and a mitotic count of >5/50 high-power fields. Various tissue specimens showed spindle cell infiltration at the junction of tumor and prostate tissue and hyperplasia of the prostate tissue. Nerve growth was found in the prostate tissue, but no necrosis or vascular invasion was observed. The final pathological diagnosis was a spindle cell tumor, possibly sarcoma. Immunohistochemistry was performed for tumor classification. The results of immunohistochemistry (Figure 2(d)–(f)) showed positive expression of CD117, DOG-1, CD34, and smooth muscle actin and negative expression of S100 and desmin, and the Ki-67LI was approximately 10%; these findings supported the diagnosis of EGIST. The patient received no adjuvant therapy after the operation. Color Doppler ultrasound of the pelvis was conducted 3 weeks later, and blood clots were detected in the bladder. Follow-up was conducted for >3 years, and no recurrence has been detected to date.
Figure 1.
Pelvic computed tomography. (a) Asymmetrical enlargement of the prostate and uneven cell density. (b) The prostate was asymmetrically enlarged and pressed against the anterior rectal wall.
Figure 2.
Pathological and immunohistochemical observations. (a–c) Biopsy-obtained pathological sections (hematoxylin–eosin; 4×, 20×, 40×). Disordered diffusion of tumor cells, mainly comprising spindle cells, was observed in a braiding shape. (d–f) Immunohistochemical examination of CD34, CD117, and DOG-1 in biopsy specimens. Positive expression of CD117, CD34, and DOG-1 was widespread among the tumor cells.
This case report was approved by the Medical Ethics Committee of the First Affiliated Hospital of Yangtze University. The patient provided written informed consent to undergo the operation and authorized us to examine the surgical specimen.
Discussion
GIST, which originates from the gastrointestinal tract, accounts for 4% to 7% of soft tissue tumors in the abdominal cavity.1,2 These soft tissue tumors originate from outside the gastrointestinal tract and have pathological characteristics, immunohistochemical biomarkers, and molecular biological characteristics similar to those of GIST. EGISTs accounts for <5% of GISTs and is more common in men aged >50 years.2,3 EGIST has been frequently reported in the mesentery, omentum, posterior peritoneum, scrotum, bladder, ovary, pancreas, and vagina, while prostatic EGIST has only been reported in a few cases.3–7 The clinical manifestations of prostatic EGIST include frequent micturition, urgent urination, odynuria, dysuria, hematuria, difficult defecation, and excrement pattern changes.3–11 Unfortunately, because of the small size of the tumor and lack of obvious symptoms, EGIST is difficult to diagnose in the early stage. Tumor growth may result in various nonspecific symptoms.5,7,11 The low incidence of prostatic EGIST and the lack of classic symptoms often result in misdiagnosis.7,11 MRI and CT are important imaging methods for the diagnosis of EGIST.11 MRI not only provides information regarding tumor growth and the connection between the tumor and adjacent tissues, but it also contributes to definition of the hematoma size, necrotic area, and diagnosis of benign from malignant disease. The diagnosis of prostatic EGIST mainly depends on intraoperative prostate biopsy and immunohistochemistry. Like GIST, EGIST is characterized by various tumor cell morphologies mainly comprising spindle cells, epithelioid cells, and polymorphic cells. As the most common cell types in EGIST tissues, spindle cells are spiral-shaped and contain tufted, light pink cytoplasm, an indistinct cell membrane, even staining, and an unclear nucleus. Epithelioid cells are round cells arranged in a rotiform and nestlike pattern with pink, clear cytoplasm and are commonly seen among EGIST cells. The cell nuclei are distributed in an eccentric manner and exhibit even staining and small nucleoli.12 Immunohistochemistry shows positivity for CD117 and CD34. The etiology of EGIST remains unclear, but it might result from uncontrolled phosphorylation and cell growth triggered by activation of gene signals due to mutations of c-kit exons (9, 11, and 13) and platelet-derived growth factor receptor alpha (PDGFRA) exons (12, 14, and 18).8 CD117 is a transmembrane receptor driven by mutated c-kit or PDGFRA and mainly exists in the cytoplasm of the cells of Cajal.13 About 81% to 100% of patients with EGIST have positive staining for CD117, which serves as a specific biomarker for EGIST. CD34 is an 11-kDa glycosylated transmembrane protein with a positive rate of 50% to 70% in patients with EGIST, making it a less sensitive biomarker for EGIST. DOG-1 is a membrane channel protein with a positive rate of 92% in patients with GIST with c-kit mutation. Compared with the CD117-positive rate of 81%, DOG-1 has higher specificity and sensitivity for GIST. Therefore, in patients with suspicious cellular morphology but with negative expression of CD117, detection of DOG-1 is commonly recommended for an accurate diagnosis.3,15 Patients with EGIST also express immune markers such as smooth muscle actin, desmin, and S-100.3 Confirmation of EGIST requires the presence of CD117-positive cells, but the dependence on a single cell biomarker is unreasonable because a minority of patients with EGIST show negativity for CD117. Similarly, tumors without the characteristics and morphologies of EGIST but with positive expression of CD117 should not be diagnosed as EGIST.12 Although EGIST and GIST have similar pathological and immunological characteristics, the incidence of EGIST is much lower than that of GIST. Therefore, in the diagnosis of EGIST, the clinician should first remove the possibility of a tumor originating from the gastrointestinal tract. GISTs mainly occur in the stomach and small intestine; a minority of GISTs originate from the rectum, a small portion of which are misdiagnosed as prostatic EGIST.7,11,15 The clinicopathological characteristics and outcomes have been presented in previous case reports (Table 1).
Table 1.
Comparison of clinicopathological characteristics and outcomes of reported cases of prostatic EGISTs Case 2 is Reference 9; Case 3 is Reference 7; Case 6 is Reference 8; Case 7 is Reference 11; Case 8 is Reference 10.
| Case No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Study (year) | Zhifei et al. (2013) | Ou et al. (2013) | Liu et al. (2014) | Wei et al. (2014) | Wei et al. (2014) | Liu et al. (2014) | Huh et al. (2014) | Zhang et al. (2014) |
| Age (years) | 31 | 39 | 50 | 29 | 56 | 55 | 50 | 31 |
| Tumor size (cm) | 6.5 | 10.0 | 8.0 | 8.0 | N/A | 10.5 | 11.0 | 9.0 |
| Immunohistochemistry | CD117(+)CD34(+)SMA(−)S100(−) | CD117(+)CD34(+)Vim(+)SMA(−)S100(−)CK(−)Desmin(−) | CD117(+)DOG-1(+)CD34(+)Vim(+)SMA(−)S100(−)CK(−) | CD117(+)CD34(+) | CD117(+)CD34(+) | CD117(+)DOG-1(+)CD34(+)Vim(+)SMA(−)S100(−)CK(−)Desmin(−) | CD117(+)CD34(+)SMA(−)S100(−)CK(−)Desmin(−) | CD117(+)CD34(+)DOG-1(+)SMA(−)S100(−) |
| Treatment | Diagnosis on biopsy, imatinib | Diagnosis on biopsy, radical prostatectomy, imatinib | Radical prostatectomy | Diagnosis on biopsy, radical prostatectomy, imatinib | Local excision, imatinib | Imatinib | Diagnosis on biopsy | Diagnosis on biopsy, imatinib |
| Follow-up interval (months) | 6 | 24 | 6 | 42 | 10 | 12 | N/A | 3 |
| Metastasis | None | None | None | None | None | None | None | None |
| Outcome | No recurrence | No recurrence | No recurrence | No recurrence | No recurrence | No recurrence | N/A | No recurrence |
SMA, smooth muscle actin; Vim, vimentin; CK, cytokeratin; N/A, not available.
The classification of benign versus malignant EGIST has not been clearly defined. Past reports have shown that malignant EGIST has the following characteristics: non-classic tumor cells, tumor necrosis, muscular infiltration, coin-shaped cell growth near blood vessels, mitotic index of ≥10/50 high-power fields, mucosal infiltration, nerve infiltration, adipose infiltration, blood vessel infiltration, and lymph node metastasis.16 In April 2008, the National Institutes of Health reached a consensus regarding the risk classification for EGIST.11,12 Assessment of the risk of recurrence of EGIST is important and beneficial for better treatment of this disease.8
Complete tumor resection is the first choice for resectable primary prostatic EGIST.3 The operation strategies for EGIST include radical prostatectomy, vesical prostatectomy, and total pelvic exenteration, among which radical prostatectomy has the best efficacy for EGIST.8 The operation strategies are dependent upon the tumor size, tumor location, and degree of infiltration, and endorectal ultrasonography-guided prostate biopsy can provide valuable information for determination of the operation strategy.3,7,8 However, surgical treatment of infiltrated or metastatic EGIST is not yet available. At present, chemotherapy for advanced EGIST and prevention of postoperative recurrent and metastatic EGIST often involves the use of tyrosine kinase inhibitors (imatinib and sunitinib), especially for GISTs with positive expression of CD117. Imatinib and sunitinib can suppress the activity of tyrosine kinase and block tyrosine kinase receptors, thus inhibiting the signal transduction.7 Muto et al.17 proposed a new strategy for invasive EGIST by combing preoperative chemotherapy and surgical resection. Although the data regarding prediction of the prognosis of prostatic GIST is limited, evidence shows that the risk of EGIST is similar to that of small intestinal GIST.8 Imaging follow-up (abdominal and pelvic cavity CT) is considered a possible strategy with which to control recurrence of prostatic EGIST.
In conclusion, prostatic EGIST is a rare disease mainly diagnosed by the combination of imaging, pathological, and immunohistochemical examinations. Detection of CD117, CD34, and DOG-1 as well as mutation analysis (c-kit and PDGFRA) is of great diagnostic value for EGIST. The diagnosis of EGIST requires ruling out tumors originating from the gastrointestinal tract. Surgical resection remains the major strategy for prostatic EGIST and exhibits satisfactory efficacy. In patients with high-risk EGIST, subtotal resection and long-term follow-up are recommended.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Sashidharan P, Matele A, Matele U, et al. Gastrointestinal stromal tumors: a case report . Oman Med J 2014; 29: 138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tornillo L. Gastrointestinal stromal tumor–an evolving concept. Front Med (Lausanne) 2014; 1: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Wei, Fang Ping, Sun Guang, et al. Two cases of primary prostatic extra-gastrointestinal stromal tumor and literature review . Shandong Medical Journal 2014; 5: 83–85. doi: 10.3969/j.issn.1002-266X.2014.05.036 [Chinese article, no English abstract]. [Google Scholar]
- 4.He F, Fang Z, Zhu P, et al. Bladder extragastrointestinal stromal tumor in an adolescent patient: a case-based review. Mol Clin Oncol 2014; 2: 960–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagkrezos D, Touloumis Z, Giannila M, et al. Extra-gastrointestinal stromal tumor of the omentum: a rare case report and review of the literature. Rare Tumors 2012; 4: e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Zhifei, Zhang Zhihong, Xu Yong, et al. Extra gastrointestinal stromal tumor of the prostate: a case report and literature review. Journal of Clinical Urology 2013; 28: 607–609. doi: 10.13201/j.issn.1001-1420.2013.08.025 [Chinese article, English abstract]. [Google Scholar]
- 7.Liu S, Yu Q, Han W, et al. Primary gastrointestinal stromal tumor of the prostate: a case report and literature review. Oncol Lett 2014; 7: 1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu S, Yu Q, Han W, et al. Primary gastrointestinal stromal tumor of the prostate: a case report and literature review[J]. Oncol Lett 2014; 7: 1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ou Z, Cao Z, He Y, et al. Diagnosis and multimodal therapy for extra gastrointestinal stromal tumor of the prostate: a case report. Exp Ther Med 2013; 6: 378–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang ZH, Feng GW, Liu ZF, et al. A young man with primary prostatic extra-gastrointestinal stromal tumor: a rare case report and review of the literature. Int J Clin Exp Pathol 2014; 7: 1764–1770. [PMC free article] [PubMed] [Google Scholar]
- 11.Huh JS, Park KK, Kim YJ, et al. Diagnosis of a gastrointestinal stromal tumor presenting as a prostatic mass: a case report. World J Mens Health, 2014, 32: 184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derek Raghavan, Martin L. Breche, David H. Johnson, et al. Textbook of uncommon cancer [M]. Third Edition The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, England; John Wiley & Sons Ltd, 2006: pp.418–428. [Google Scholar]
- 13.Corless CL. Gastrointestinal stromal tumors: what do we know now? Mod Pathol 2014; 27: S1–S16. [DOI] [PubMed] [Google Scholar]
- 14.Wada T, Tanabe S, Ishido K, et al. DOG1 is useful for diagnosis of KIT-negative gastrointestinal stromal tumor of stomach. World J Gastroenterol 2013; 19: 9133–9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi JH, Sim J, Park BB, et al. The primary extra-gastrointestinal stromal tumor of pleura: a case report and a literature review. Jpn J Clin Oncol 2013; 43: 1269–1272. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Sang M, Mancho S, Tsang AW, et al. A malignant omental extra-gastrointestinal stromal tumor on a young man: a case report and review of the literature. World J Surg Oncol 2008; 6: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muto M, Fujiya M, Okada T, et al. An invasive extragastrointestinal stromal tumor curably resected following imatinib treatment. J Gastrointestin Liver Dis 2013; 22: 329–332. [PubMed] [Google Scholar]