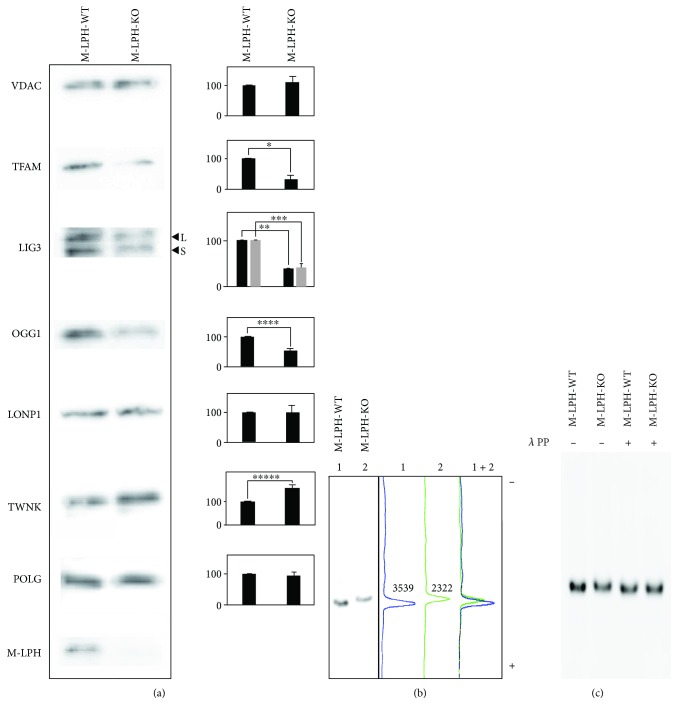
Figure 9.
Western blot analysis of mitochondrial extracts from M-LPH-WT and -KO HepG2 cells. (a) The intramitochondrial levels of proteins essential for mtDNA stability and maintenance were examined using SDS-PAGE gels. Analysis was performed using the membrane extract (ME) used in Figure 2(b). VDAC was used as a loading control for mitochondrial protein. L and S in the figure correspond to the two mitochondrial isoforms of LIG3 generated by the alternative splicing. Three preparations of the membrane extract from WT and KO cells, respectively, were used for analysis. The bars represent the mean ± SD of the results from three independent experiments. Differences at p < 0.05 were considered to be statistically significant (∗p = 0.016, ∗∗p = 0.00018, ∗∗∗p = 0.0075, ∗∗∗∗p = 0.0091, and ∗∗∗∗∗p = 0.03). (b) Western blot analysis using Zn2+-Phos-tag gels was performed to investigate the state of phosphorylation of TFAM. In Zn2+-Phos-tag gel electrophoreses, phosphorylated proteins exhibit lower electrophoretic mobility than do corresponding dephosphorylated proteins [27]. In the presence of 50 μM Phos-tag, the Rf values for TFAM band in M-LPH-KO cells were smaller than those in M-LPH-WT cells. Numbers in graphs represent the intensity of each band. (c) Mitochondrial extracts from M-LPH-WT and -KO cells were incubated with or without 40 U/μl of λ protein phosphatase and analyzed by Zn2+-Phos-tag SDS-PAGE followed by immunoblotting with the anti-TFAM antibody.