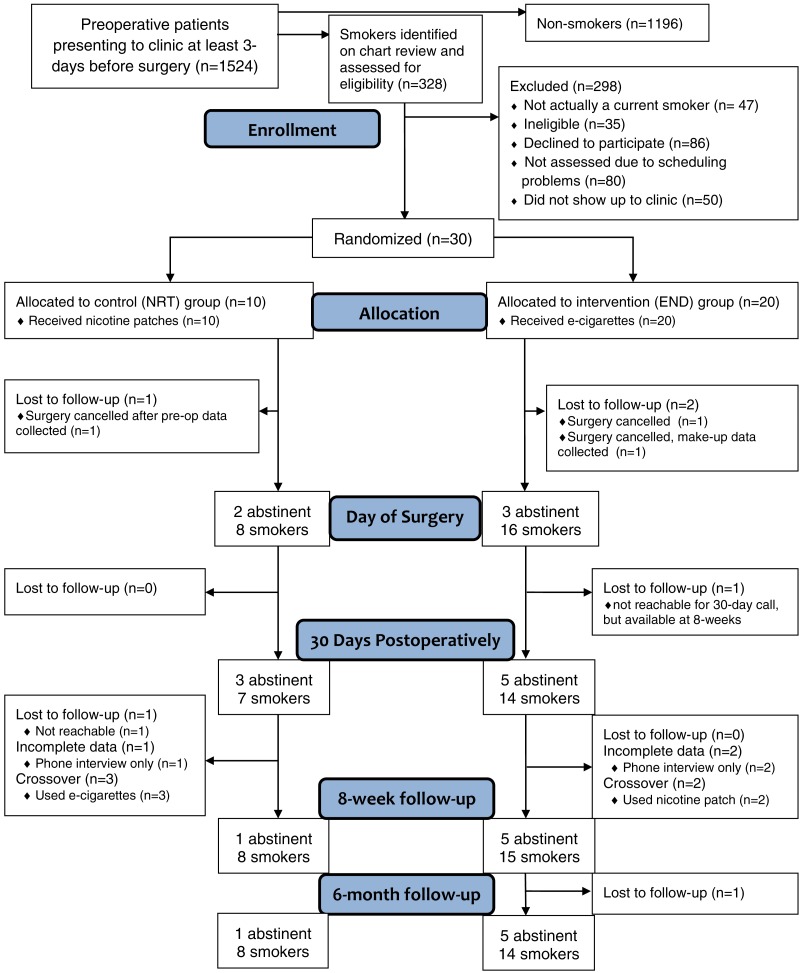
Figure 1. Consolidated Standards of Reporting Trials flow chart indicating recruitment, randomization and retention of trial participants.
Of the 35 patients approached for inclusion but found to be ineligible, the reasons for ineligibility included: smoking less than two cigarettes per day (n = 10), already being on smoking cessation pharmacotherapy (n = 9), smoking non-cigarette tobacco only (n = 5), prior adverse reaction to NRT patch (n = 3), already enrolled in smoking cessation program (n = 3), regular use of e-cigarettes (n = 2), surgical date changed (n = 2) and currently experiencing an unstable cardiac condition (n = 1). One patient was found to be ineligible after consent, but prior to randomization. All patients were given the treatment (END or NRT) to which they were randomized. Losses to follow-up were minimal and balanced between groups.