Figure 1. Protein C competes with FVIIa and FVIIa-DVQ for platelet binding in an EPCR-dependent manner.
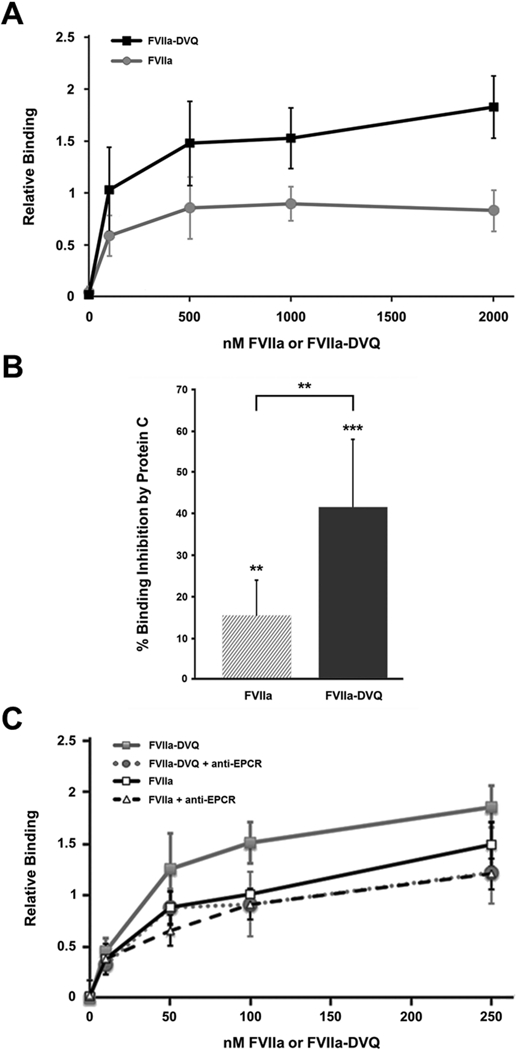
(A) Platelets activated with thrombin plus convulxin were incubated with the indicated concentrations of FVIIa or FVIIa-DVQ. The platelet binding of each molecule was analyzed by flow cytometry as described. Binding was normalized to the highest level of FVIIa binding in each experiment (n=3). (B) Similar experiments were performed to determine the platelet binding of 100nM FVIIa or FVIIa-DVQ in the presence and absence of excess (250nM) Protein C. Data shown represents the mean ± SD (n=7). (C) The platelet binding of various concentrations of FVIIa or FVIIa-DVQ was similarly analyzed in the presence and absence of an anti-EPCR antibody (blocking). Binding was normalized to the binding of FVIIa at 100 nM (=100%). No difference was seen in the binding of either molecule in the presence of an isotype control antibody. Data shown represents the mean ± SD (n=3). ** P < 0.01, *** P < 0.001 by paired t-test.
