Abstract
Background
ABO-incompatible (ABOi) living donor liver transplantation (LDLT) has been performed to compensate for donor shortage. To date, few studies have reported detailed B-cell desensitization protocols and long-term outcomes of ABOi pediatric LDLT.
Methods
Twenty-nine pediatric ABOi LDLT recipients were retrospectively analyzed. We compared the clinical outcomes between ABOi (n = 29) and non-ABOi (n = 131) pediatric LDLT recipients. Furthermore, we evaluated the safety and efficacy of our rituximab-based regimen for ABOi pediatric LDLT (2 ≤ age < 18; n = 10).
Results
There were no significant differences in the incidence of infection, vascular complications, biliary complications, and acute cellular rejection between ABOi and non-ABOi groups. The cumulative graft survival rate at 1, 3, and 5 years for non-ABOi group were 92.1%, 87.0%, and 86.1%, and those for ABOi group were 82.8%, 82.8%, and 78.2%, respectively. Rituximab-based desensitization protocol could be performed safely, and reduced CD19+ lymphocyte counts effectively. Although rituximab-treated ABOi group showed comparable clinical outcomes and graft survival rate, 2 patients developed antibody-mediated rejection.
Conclusions
ABOi LDLT is a feasible option for pediatric end-stage liver disease patients. However, it should be noted that current desensitization protocol does not completely prevent the onset of antibody-mediated rejection in several cases.
The authors of this single-center retrospective study demonstrate that ABO incompatible (ABOi) LDLT is a feasible option for pediatric end-stage liver disease patients by comparing the clinical outcomes between ABOi and non-ABOi pediatric LDLT recipients as well as assessing the safety and efficacy of a rituximab-based desensitization protocol. Supplemental digital content is available in the text.
Liver transplantation has been established as an effective treatment for end-stage liver disease. Although considerable progress of perioperative care and the refinement of surgical techniques have been achieved, chronic donor shortage has been a serious problem globally. In several countries, living donor liver transplantation (LDLT) remains a major modality because of the limited availability of deceased donor organs for sociocultural reasons. Furthermore, liver grafts from ABO-incompatible (ABOi) donors have been used to increase the possibilities of transplantation.1,2 Pediatric patients who suffer from liver disease are no exception to this issue.
Advanced strategies in ABOi LDLT through innovation of B-cell desensitization using intravenous immunoglobulin, intrahepatic portal and/or arterial infusion therapy, plasma exchange (PE), splenectomy, and anti-CD20 monoclonal antibody, rituximab have expanded the donor pool effectively over the last 2 decades.3-7 Indeed, recent studies using the induction of rituximab have demonstrated the dramatically improved survival rate in ABOi pediatric and adult LDLT.1,8-11 However, the incidence of antibody-mediated rejection (AMR) remains an issue that has not yet been completely resolved. In addition, several reports showed that concerns still remain in the incidence of high prevalence rates, such as biliary stricture and infectious complications.1,8,12
To date, few studies have reported the detailed B-cell desensitization protocol and long-term outcomes of ABOi pediatric LDLT in rituximab era. Therefore, safety and efficacy of the rituximab based protocol for ABOi pediatric LDLT are unclear. The aims of our single-center study are to analyze the long-term outcomes of pediatric patients who underwent ABOi LDLT and to assess the adequacy of the immunosuppressive protocol against the ABO-barrier.
MATERIALS AND METHODS
Study Population
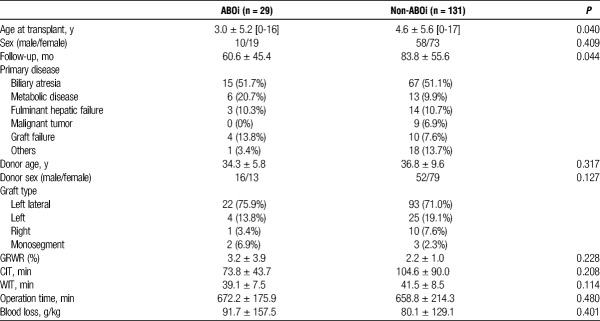
Between December 1998 and March 2016, 444 patients underwent 463 LDLT at Kumamoto University Hospital. Of these, 160 pediatric LDLT recipients younger than 18 years were analyzed retrospectively by reviewing the clinical records. Data were collected and analyzed in December 2016. All patients were of Asian ethnic origin. In the study population, 29 recipients underwent ABOi LDLT. The characteristics of study population are shown in Table 1. All our LDLT protocol received an approval from the institutional review committee. This study was performed according to the Ethical Guidelines for Clinical Research published on April 1, 2009, by the Ministry of Health, Labour and Welfare of Japan.
TABLE 1.
Patients characteristics (n = 160)
Surgical Procedure
The transplant procedures in our institution have been described previously.13,14 Briefly, hepatic and portal veins were reconstructed under a surgical loupe, and hepatic arteries were reconstructed under a microscope. Duct-to-duct biliary reconstruction was a routine procedure except for the recipients with biliary atresia. Absolute indication of graft size reduction was that the estimated graft-to-recipient weight ratio (GRWR) was greater than 4.0%. Even if the preoperative GRWR was less than 4.0%, the reduction was considered to ensure a size match.
Basic Immunosuppressive Regimen
The immunosuppressive regimen consisted of tacrolimus combined with low-dose steroids. The target trough levels of tacrolimus were between 10 and 15 ng/mL in the first 2 weeks, around 10 ng/mL in the next 2 weeks, and between 5 and 10 ng/mL thereafter. Steroids were initiated with an injection of 10 mg/kg of methylprednisolone before graft perfusion during surgery. Recipients received the intravenous injection of 1 mg/kg of methylprednisolone during postoperative day (POD) 1-3, 0.5 mg/kg during POD 4-6, and 0.3 mg/kg at POD 7. Subsequently, they were changed to oral administration of prednisolone and were tapered off until around 3 to 6 months. When acute cellular rejection (ACR) was suspected by a liver function test, patients were initially treated by increasing the dose of tacrolimus. If a liver function test showed no improvement or ACR as proven by liver biopsy, high-dose methylprednisolone (10 mg/kg) was administered at 3 days as a steroid pulse therapy and then the dose was tapered (over 7-10 days totally).
Immunosuppressive Protocol for ABOi Pediatric LDLT
We have performed a total of 29 cases of ABOi pediatric LDLT, of which 10 cases were 2 years or older. A target trough level of tacrolimus was the same as that of non-ABOi cases as described above. Steroids were administered as same as non-ABOi cases until 1 month after LDLT, and tapered off taking twice as much time. Oral administration of mycophenolate mofetil (MMF) (10 mg/kg twice a day) was started POD 1. In patients younger than 2 years, preoperative PE was performed in 6 of 8 cases by 2010 to decrease the antidonor blood group antibody to less than 16, and in 11 cases since 2010, additional prophylaxis protocol was not performed (Figure 1). All of patients who were 2 years or older received single dose of rituximab 2 weeks before LDLT (3 of them received at different timings). The dose of rituximab was 300 mg/m2 for 1 patient, 375 mg/m2 for 6 patients, and 500 mg/body for 3 patients. Of these, the first consecutive three patients received additional B-cell desensitization using preoperative PE, infusion therapy, and splenectomy. From 2010, we have used a protocol without additional B-cell desensitization except for rituximab. Acetaminophen (10 mg/kg) and d chlorpheniramine maleate (0.04 mg/kg) were orally administered before administration of rituximab to prevent adverse events. The diagnosis of AMR was made based on the clinical course, immunological assays, and histopathological findings.15,16 C4d immunostaining was performed case by case if AMR was suspected.17
FIGURE 1.
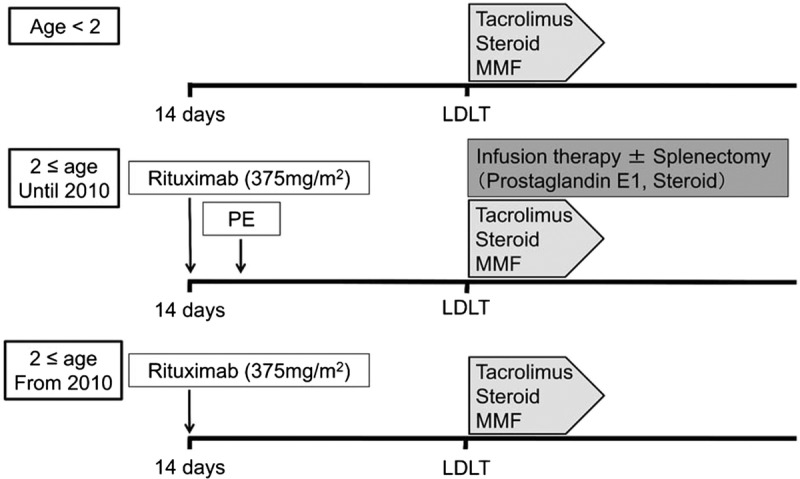
Protocol of the pediatric ABOi LDLT at Kumamoto University Hospital.
Evaluated Factors
As patient characteristics, clinical data including age at transplant, sex, primary disease, donor age, donor sex, graft type, GRWR, blood loss, cold ischemia time (CIT), warm ischemia time (WIT), and operation time were assessed. Moreover, incidence for bacterial infection, cytomegalovirus infection, fungal infection, vascular complication, biliary complication, ACR, AMR, and graft survival were compared between ABOi and non-ABOi LDLT group. Bacterial infection was defined as elevated inflammatory parameters accompanied by infected foci, and cytomegalovirus infection was evaluated by the antigenemia test. Fungal infection was diagnosed with identification in culture or image findings accompanied by the increase of β-d glucan. Vascular and biliary complications were defined as those requiring some intervention. Rituximab-treated group was further evaluated in detail including pediatric end-stage liver disease (PELD) score (under 12 years of age) or model for end-stage liver disease score, blood type combination, lymphocyte crossmatch test (flow cytometry), peak titer of Immunoglobulin M (IgM) and G (IgG) isoagglutinin against donor erythrocyte antigens at admission, at LDLT, and after transplantation, proportion of CD19+ lymphocyte cells (%) before rituximab treatment and at LDLT, and adverse events of rituximab treatment. The clinical course before and after AMR onset was described separately in detail.
Statistical Analysis
Continuous variables were expressed as mean values ± standard deviations. Statistical analysis was performed using the Mann-Whitney U test or Wilcoxon signed-rank test as appropriate for continuous data, whereas categorical variables were compared using either the χ2 test (without the Yates correction) or Fisher exact test as appropriate. The cumulative graft survival was estimated using the Kaplan-Meier product limit method. The log-rank (Mantel-Cox) test was used for comparison of the curves. A P value less than 0.05 was considered statistically significant; all tests were 2-tailed. All statistical analyses were performed using the GraphPad Prism 7 (GraphPad Software).
RESULTS
Clinical Characteristics
Among 160 pediatric LDLT recipients, 29 recipients underwent ABOi LDLT (Table 1). The mean age at transplant was significantly lower in ABOi group (3.0 ± 5.2 years vs 4.6 ± 5.6 years, P = 0.040). The mean follow-up period was 60.6 ± 45.4 months in ABOi group and was 83.8 ± 55.6 months in non-ABOi group. The most common indication for ABOi LDLT was biliary atresia (51.7%). There were no significant differences in donor-related factors including donor age and sex between ABOi and non-ABOi groups. The most used graft type was left lateral (75.9%), and mean GRWR was 3.2 ± 3.9 in ABOi group. Operation-related factors including graft type, GRWR, CIT, WIT, operation time, and blood loss also showed no significant differences.
Postoperative Outcomes and Graft Survivals
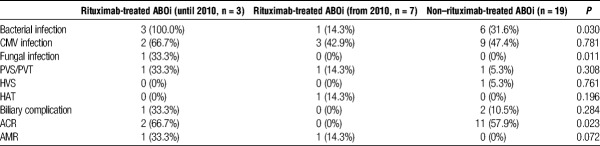
There were no significant differences in the incidence of infection, vascular complications, biliary complications, and ACR between ABOi and non-ABOi group (Table 2). Meanwhile, 2 (6.9%) patients developed AMR in ABOi group but not in non-ABOi group (P = 0.036). Next, to elucidate the impact of rituximab treatment, we compared the clinical outcomes between rituximab-treated and non–rituximab-treated ABOi group. Rituximab-treated 10 patients were classified into 2 groups based on the additional B-cell desensitization protocol as described. Reflecting excessive immunosuppression, rituximab-treated ABOi group (until 2010, n = 3) showed a higher incidence of bacterial and fungal infection compared with rituximab-treated ABOi group (from 2010, n = 7) and non–rituximab-treated ABOi group (n = 19) (Table 3). The cumulative graft survival rate at 1, 3, and 5 years for non-ABOi group were 92.1%, 87.0%, and 86.1%, respectively, and those for ABOi group were 82.8%, 82.8%, and 78.2%, respectively (P = 0.375, Figure 2A). Rituximab-treated ABOi group showed comparable graft survival rate (80.0%, 80.0%, 66.7% at 1, 3, and 5 years with a mean follow-up of 40.7 ± 27.4 months) compared with the non-ABOi and non–rituximab-treated ABOi group (P = 0.328, Figure 2B). During the study period, mortality rate in non–rituximab-treated ABOi group was 15.8%, and in rituximab-treated ABOi group was 30.0%. The cause of death in the former group was heart failure (in related to the primary disease, glycogen storage disease type IV), pulmonary hypertension (in related to the primary disease, mitochondrial DNA depletion syndrome), and interstitial pneumonia (Table S1, SDC, http://links.lww.com/TP/B555). The cause of death in the latter group was AMR in 2 patients and the other was multiple organ failure related to complications of the primary disease (progressive familial intrahepatic cholestasis type 1 [PFIC1]) including bleeding from the ileostomy, severe malnutrition, and chronic renal failure.18
TABLE 2.
Clinical outcomes between ABOi and non-ABOi group
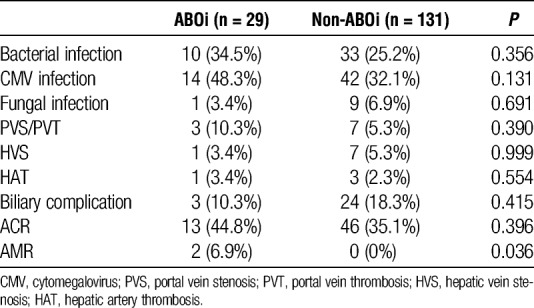
TABLE 3.
Clinical outcomes between rituximab-treated and non–rituximab-treated ABOi group
FIGURE 2.
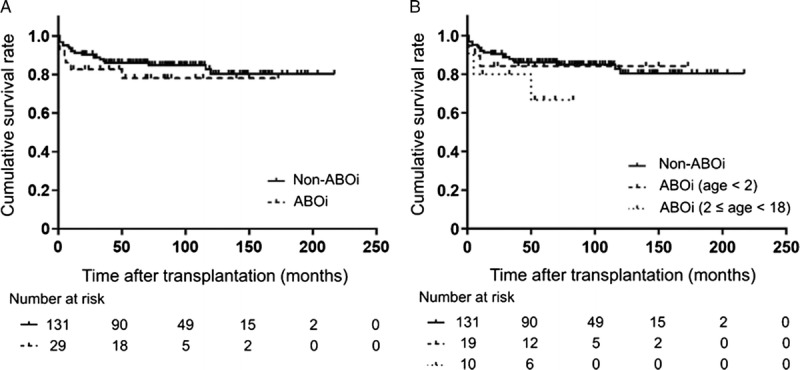
Cumulative graft survival rate of pediatric LDLT recipients. (A) Comparison between ABOi and non-ABOi group. Log-rank test, P = 0.375. (B) Comparison between ABOi (age < 2), ABOi (2 ≤ age < 18), and non-ABOi group. Log-rank test, P = 0.328.
Safety and Efficacy of Rituximab for ABOi Pediatric LDLT
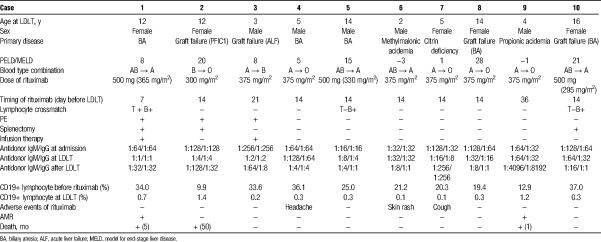
To analyze the detailed clinical course in related to the rituximab administration for pediatric patients, we reviewed the 10 rituximab-treated ABOi LDLT recipients whose age was 2 years or older (Table 4). Mean age at LDLT was 8.6 ± 5.5 and male-female ratio was equivalent. The most common indication for rituximab-treated ABOi LDLT was graft failure (40.0%). The original disease was PFIC1 in case 2 (104 months from first LDLT), acute liver failure in case 3 (36 months from first LDLT), and biliary atresia in cases 8 and 10 (163 and 192 months from first LDLT, respectively). The cause of graft failure was chronic rejection in cases 2, 3, 8, and unknown in case 10. Cases 1 to 3 had received PE and splenectomy and/or infusion therapy during the perioperative period. The lymphocyte crossmatch test was positive for T and B cells in case 1, and for B cells in cases 5 and 10. The median titers of IgM and IgG isoagglutinin at LDLT were 1:16 (1:1–1:256), 1:8 (1:1–1:256), respectively. CD19+ lymphocyte counts were decreased significantly after rituximab administration (24.9 ± 9.8% to 0.49 ± 0.46%, P < 0.001). Case 2 experienced rebound elevation of the CD19+ lymphocyte counts (19.4%) and received 375 mg/m2 rituximab 10 days after LDLT. When rituximab was administered, 1 case of headache, rash, cough was observed, but both of symptoms were mild, and it was not necessary to stop the treatment. In addition, although significant elevation of body temperature was observed after rituximab treatment (36.7 ± 0.4°C to 37.6 ± 0.4°C, P < 0.001), which improved promptly within 1 day.
TABLE 4.
Detailed outcomes of rituximab-treated ABOi pediatric LDLT patients
In rituximab-treated group, 2 patients (cases 1 and 9) developed AMR. In case 1, both T and B were positive in the preoperative lymphocyte crossmatch test. Although the elevation of antidonor type IgM and IgG could not be observed after LDLT, the patient repeated cholangitis from the early stage of posttransplant and showed the distinctive AMR phenotype with intrahepatic biliary complications. The patient received high-dose intravenous immunoglobulin administration and percutaneous transhepatic biliary drainage against multiple biloma, however eventually died of graft failure 5 months after LDLT. As previously reported, case 9 developed streptococcal infection 13 days after rituximab, and LDLT was postponed.19 CD19+ lymphocyte count decreased to 0.1% at 9 days after rituximab administration, but the number just before LDLT increased to 1.2%. The patient showed an increased ascites, a marked increase of hepatic enzyme levels, and decreased platelet levels on POD 5. Both of the IgM and IgG antidonor antibody titers increased to 1:1024, and the CD19+ lymphocyte count increased to 4.1%. The liver histology showed hepatocyte ballooning, portal inflammation, sinusoidal congestion, and complement component 4d positivity in the vascular endothelium. Therefore, in this case, it was speculated that streptococcal infection resulted in reactivation of B cells, which might make a foothold to trigger AMR. Despite treatment with PE, intravenous immunoglobulin, steroid pulse therapy, and readministration of rituximab, the patient died with graft failure accompanied by renal failure and acute respiratory distress syndrome 1 month after LDLT.
DISCUSSION
In Japan, ABOi grafts have been used in 13.8% of pediatric LDLT from 1989 to 2013.20 Thus, even in pediatric patients, ABOi LDLT has been implemented as an important option to compensate for the shortage of donors. In our institution, 18.1% of pediatric LDLT was performed using ABOi grafts and their clinical outcomes and graft survival were comparable to non-ABOi grafts. We evaluated the clinical outcomes and the adequacy of the immunosuppressive protocol in ABOi pediatric LDLT.
Previous literatures have reported that infants show better outcomes in ABOi LT.2,21 One possible reason is the different immune responses of infants to ABOi graft. Infants do not produce isohemagglutinins, therefore, their anti-A and -B antibody titers remain low levels in early childhood.22 Additionally, the activation of complement system is suppressed in infants.23 Taken together, infants have less mediators in related to the AMR. In accordance with these mechanisms, ABOi pediatric LDLT recipients who are younger than 2 years in our study cohort did not develop AMR. Therefore, we believe that it is unnecessary to use rituximab in ABOi pediatric LDLT younger than 2 years.
We have used the rituximab-based protocol in 10 pediatric ABOi LDLT recipients for B-cell desensitization. The pretransplant therapy could be performed safely without severe adverse events and reduced their CD19+ lymphocyte counts effectively just before LT. However, regardless of the desensitization, we experienced 2 cases (cases 1 and 9) of AMR. Case 1 developed the distinctive AMR phenotype with intrahepatic biliary complications without the elevation of anti-donor type IgM and IgG. Characteristically, case 1 had shown positive lymphocyte crossmatch for T and B cells before LDLT. Importantly, Hori et al24 have shown that a positive lymphocyte crossmatch has a negative impact on LDLT possibly because of the wide expression of HLA antigens. As such background might affect clinical course of case 1, advanced immunological strategies must be considered for lymphocyte crossmatch-positive recipients. Case 9 showed specific clinical course suggesting the streptococcal infection after rituximab administration resulted in reactivation of B cells, which might trigger AMR.19 In the light of experience, we think that additional desensitization therapy should be considered if the reactivation of B cells is suspected before ABOi LDLT.
Plasmapheresis is a standard procedure to reduce donor specific antibody titers, but the titer required to prevent AMR is not defined. Egawa et al1 observed no significant relationships between plasmapheresis and clinical outcomes after ABOi adult LDLT from a Japanese multicenter study. The study also revealed that local infusion, splenectomy, antilymphocyte antibody, and intravenous immunoglobulin had no significant impact on overall survival or AMR incidence in rituximab-treated ABOi LDLT recipients. As these results indicate, we believe that the most important key to prevent AMR in ABOi LT is inhibition of new antibody production. Additionally, the procedure, such as catheter insertion for local infusion, or splenectomy increases the risk of bleeding and infection. Based on this policy, we have used a protocol based on the administration of rituximab (patients who were 2 years or older), tacrolimus, steroids, and MMF without PE, local infusion, and splenectomy since 2010 in ABOi LDLT.25
From the viewpoint of rituximab dose, it is widely accepted that a single maximum dose of rituximab with efficacy and safety is 375 mg/m2 for the B-cell lymphoma treatment.26 Recently, Egawa et al27 suggested that the dose in 300 mg/m2 or less of rituximab single administration would be insufficient for prevention of AMR in ABOi adult LDLT. Indeed, 1 patient who had received 300 mg/m2 of rituximab showed rebound elevation of the CD19+ lymphocyte counts after LDLT in our study cohort. Thus, the use of rituximab at sufficient dose is recommended, whereas it is worth mentioning that careful attention must be paid to the prevention of infectious diseases.
The optimal treatment strategy for AMR after LDLT remains unclear so far. Based on a combination of calcineurin inhibitor, corticosteroid, plasmapheresis, and B cell–modulating therapies, use of thymoglobulin is also considered as an option to disrupt key T-cell and B-cell interactions.28 In addition, in some cases, AMR can be induced by isohemagglutinin production from plasma cells which do not express CD20. In such a case, treatment with bortezomib, which is a proteasome inhibitor, can be considered as another option.29,30 Recently, eculizumab, which is a monoclonal antibody that blocks the complement pathway, is successfully used for the treatment of AMR after pediatric LT.31 In this report, eculizumab was used for recipient showing refractory AMR associated with C1q-binding donor specific antibody. Of course, retransplantation that does not miss the time should always be considered.
The precise role of donor-specific antibodies (DSA) after LT is unclear, whereas evidence is increasing that DSA, especially those with higher mean fluorescence intensity, are associated with both acute and chronic liver allograft rejections.32-35 In our study cohort, case 1 in rituximab-treated ABOi group developed AMR which did not seem to be involved in antiblood type antibodies, therefore, involvement of DSA should be taken into consideration as the cause of AMR. For the impact of DSA on humoral immunity in post-LT follow-up, more detailed investigation will be needed, including intervention of immunosuppressive protocols. It is also worth noting that detrimental aspect of DSA might differ between deceased donor LT and LDLT.36
It has been well accepted that hypogammaglobulinemia is a crucial risk factor for development of infection.37 As a reminder, rituximab and immunosuppressive drugs, such as steroids and MMF, are known to induce iatrogenic hypogammaglobulinemia. Although ABOi pediatric LDLT recipients did not develop the recurrent infections related low IgG levels in our cohort, serum IgG levels, in particular, rituximab-treated ABOi LDLT recipients, should be monitored at least until the recovery of B cells. Proper IgG supplementation has to be done in case a serum IgG level is below 500 mg/dL with recurrent or severe infections.
Our study has some limitations. First, it was a retrospective single-center cohort study of a relatively small patient population. However, we believe that the data from our cohort are reliable because the treatment practices, immunosuppressive strategy, and surgical techniques are standardized. Prospective and multicenter studies are needed to clarify the feasibility of our protocol. Second, the observation period in ABOi group, in particular in rituximab-treated group, is shorter compared with the non-ABOi group. This difference is affected by the time of approval of rituximab, but we believe that an observation period of over 40 months on average is sufficient to evaluate the long-term posttransplant outcomes. Third, our study population does not contain acute liver failure patients in rituximab-treated group. The timing of rituximab administration is known to be related to the rebound elevation of isohemagglutinin titers,38 therefore, further investigation will be needed to detect the minimal time interval from rituximab administration to ABOi LDLT.
In conclusion, ABOi LDLT is a feasible option for PELD patients. Rituximab-based protocol is a promising procedure for preventing AMR in ABOi pediatric LDLT recipients who are 2 years or older. However, we need to keep in mind that the current protocol does not completely prevent the onset of AMR in several cases, and further research is required in the future.
Supplementary Material
Footnotes
The authors declare no funding or conflicts of interest.
M.H., Y.S., H.Y, and Y.I. participated in the study concept and design, analysis and interpretation of data, and critical revision of the article. M.H., M.K., S.K., M.S., D.Y., K.U., S.H., Y.O., H.Y., and H.Y. participated in data collection. M.H., T.H., and Y.S. participated in the writing of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Egawa H, Teramukai S, Haga H, et al. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014;14:102–114. [DOI] [PubMed] [Google Scholar]
- 2.Stewart ZA, Locke JE, Montgomery RA, et al. ABO-incompatible deceased donor liver transplantation in the United States: a national registry analysis. Liver Transpl. 2009;15:883–893. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe M, Shimazu M, Wakabayashi G, et al. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002;73:1959–1961. [DOI] [PubMed] [Google Scholar]
- 4.Egawa H, Teramukai S, Haga H, et al. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143–152. [DOI] [PubMed] [Google Scholar]
- 5.Ikegami T, Taketomi A, Soejima Y, et al. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009;88:303–307. [DOI] [PubMed] [Google Scholar]
- 6.Soejima Y, Muto J, Matono R, et al. Strategic breakthrough in adult ABO-incompatible living donor liver transplantation: preliminary results of consecutive seven cases. Clin Transplant. 2013;27:227–231. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Kwon CH, Joh JW, et al. ABO-incompatible living donor liver transplantation is suitable in patients without ABO-matched donor. J Hepatol. 2013;59:1215–1222. [DOI] [PubMed] [Google Scholar]
- 8.Song GW, Lee SG, Hwang S, et al. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61:575–582. [DOI] [PubMed] [Google Scholar]
- 9.Song GW, Lee SG, Hwang S, et al. ABO-incompatible adult living donor liver transplantation under the desensitization protocol with rituximab. Am J Transplant. 2016;16:157–170. [DOI] [PubMed] [Google Scholar]
- 10.Okada N, Sanada Y, Hirata Y, et al. The impact of rituximab in ABO-incompatible pediatric living donor liver transplantation: the experience of a single center. Pediatr Transplant. 2015;19:279–286. [DOI] [PubMed] [Google Scholar]
- 11.Rana A, Kueht ML, Nicholas SK, et al. Pediatric liver transplantation across the ABO blood group barrier: is it an obstacle in the modern era? J Am Coll Surg. 2016;222:681–689. [DOI] [PubMed] [Google Scholar]
- 12.Bang JB, Kim BW, Kim YB, et al. Risk factor for ischemic-type biliary lesion after ABO-incompatible living donor liver transplantation. World J Gastroenterol. 2016;22:6925–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okajima H, Inomata Y, Asonuma K, et al. Duct-to-duct biliary reconstruction in pediatric living donor liver transplantation. Pediatr Transplant. 2005;9:531–533. [DOI] [PubMed] [Google Scholar]
- 14.Shirouzu Y, Ohya Y, Hayashida S, et al. Reduction of left-lateral segment from living donors for liver transplantation in infants weighing less than 7 kg: technical aspects and outcome. Pediatr Transplant. 2010;14:709–714. [DOI] [PubMed] [Google Scholar]
- 15.Haga H, Egawa H, Shirase T, et al. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004;10:16–27. [DOI] [PubMed] [Google Scholar]
- 16.Demetris AJ, Bellamy C, Hubscher SG, et al. 2016 Comprehensive update of the banff working group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant. 2016;16:2816–2835. [DOI] [PubMed] [Google Scholar]
- 17.Haga H, Egawa H, Fujimoto Y, et al. Acute humoral rejection and C4d immunostaining in ABO blood type-incompatible liver transplantation. Liver Transpl. 2006;12:457–464. [DOI] [PubMed] [Google Scholar]
- 18.Oya Y, Sugawara Y, Honda M, et al. Living donor liver transplantation for progressive familial intrahepatic cholestasis type 1: two reported cases. Transplant Proc. 2017;49:1123–1125. [DOI] [PubMed] [Google Scholar]
- 19.Honda M, Sakamoto S, Sakamoto R, et al. Antibody-mediated rejection after ABO-incompatible pediatric living donor liver transplantation for propionic acidemia: a case report. Pediatr Transplant. 2016;20:840–845. [DOI] [PubMed] [Google Scholar]
- 20.Umeshita K, Inomata Y, Furukawa H, et al. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res. 2016;46:1171–1186. [DOI] [PubMed] [Google Scholar]
- 21.Egawa H, Oike F, Buhler L, et al. Impact of recipient age on outcome of ABO-incompatible living-donor liver transplantation. Transplantation. 2004;77:403–411. [DOI] [PubMed] [Google Scholar]
- 22.Fong SW, Qaqundah BY, Taylor WF. Developmental patterns of ABO isoagglutinins in normal children correlated with the effects of age, sex, and maternal isoagglutinins. Transfusion. 1974;14:551–559. [DOI] [PubMed] [Google Scholar]
- 23.Ferriani VP, Barbosa JE, de Carvalho IF. Serum haemolytic classical and alternative pathways of complement in infancy: age-related changes. Acta Paediatr Scand. 1990;79:322–327. [DOI] [PubMed] [Google Scholar]
- 24.Hori T, Uemoto S, Takada Y, et al. Does a positive lymphocyte cross-match contraindicate living-donor liver transplantation? Surgery. 2010;147:840–844. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto H, Uchida K, Kawabata S, et al. Feasibility of monotherapy by rituximab without additional desensitization in ABO-incompatible living donor liver transplantation. Transplantation. 2017. [DOI] [PubMed] [Google Scholar]
- 26.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: clinical and molecular evaluation. Blood. 2001;97:101–106. [DOI] [PubMed] [Google Scholar]
- 27.Egawa H, Umeshita K, Uemoto S. Optimal dosage regimen for rituximab in ABO-incompatible living donor liver transplantation. J Hepatobiliary Pancreat Sci. 2017;24:89–94. [DOI] [PubMed] [Google Scholar]
- 28.Hogen R, DiNorcia J, Dhanireddy K. Antibody-mediated rejection: what is the clinical relevance? Curr Opin Organ Transplant. 2017;22:97–104. [DOI] [PubMed] [Google Scholar]
- 29.Paterno F, Shiller M, Tillery G, et al. Bortezomib for acute antibody-mediated rejection in liver transplantation. Am J Transplant. 2012;12:2526–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee CF, Eldeen FZ, Chan KM, et al. Bortezomib is effective to treat acute humoral rejection after liver transplantation. Transplant Proc. 2012;44:529–531. [DOI] [PubMed] [Google Scholar]
- 31.Wozniak LJ, Naini BV, Hickey MJ, et al. Acute antibody-mediated rejection in ABO-compatible pediatric liver transplant recipients: case series and review of the literature. Pediatr Transplant. 2017;21. [DOI] [PubMed] [Google Scholar]
- 32.Kasahara M, Kiuchi T, Takakura K, et al. Postoperative flow cytometry crossmatch in living donor liver transplantation: clinical significance of humoral immunity in acute rejection. Transplantation. 1999;67:568–575. [DOI] [PubMed] [Google Scholar]
- 33.Musat AI, Agni RM, Wai PY, et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am J Transplant. 2011;11:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Leary JG, Kaneku H, Susskind BM, et al. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection postliver transplant. Am J Transplant. 2011;11:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wozniak LJ, Hickey MJ, Venick RS, et al. Donor-specific HLA antibodies are associated with late allograft dysfunction after pediatric liver transplantation. Transplantation. 2015;99:1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levitsky J, Kaneku H, Jie C, et al. Donor-specific HLA antibodies in living versus deceased donor liver transplant recipients. Am J Transplant. 2016;16:2437–2444. [DOI] [PubMed] [Google Scholar]
- 37.Compagno N, Malipiero G, Cinetto F, et al. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egawa H, Ohmori K, Haga H, et al. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579–588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.