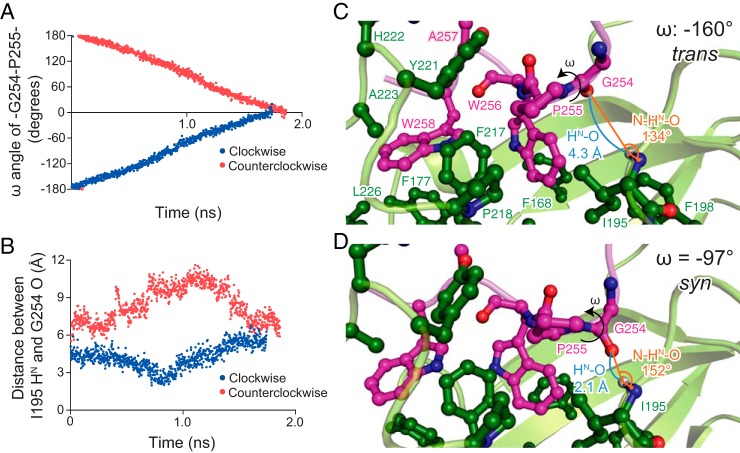
Figure 4.
Molecular dynamics simulation of cis/trans isomerization by TFPPD. A, plots of the ω dihedral angle for the peptidyl–prolyl bond between MBP Gly254 and Pro255. The simulation was started from the NMR structure of MBP–TFPPD complex in which the ω angle for the peptidyl–prolyl bond between MBP Gly254 and Pro255 is −174° (trans state). Because of the ω angle constraint at a rate of 0.2° per 2 ps, the ω angle changed linearly as a function of time. B, plots of the distance between the HN atom of TF Ile195 and the carbonyl oxygen of MBP Gly254 as a function of time when the ω angle was rotated clockwise (blue) or counterclockwise (red). When the ω angle was rotated in clockwise direction, the two atoms approached each other, and the distance became shorter than 2.5 Å at ∼0.8 ns. As seen in A, the ω dihedral angle at 0.8 ns was at approximately −90° (syn state). C and D, snap shots of the cis/trans isomerization at the trans state (0.200 ns) (C) and the syn state (0.794 ns) (D). The intermolecular hydrogen bond between backbone amide group of TFPPD Ile195 and the backbone carbonyl oxygen of MBP Gly254 is formed at the syn state, which tethers the peptidyl–prolyl bond closer onto the hydrophobic cleft formed by TFPPD Ile195, Phe217, and Pro218.