Figure 6.
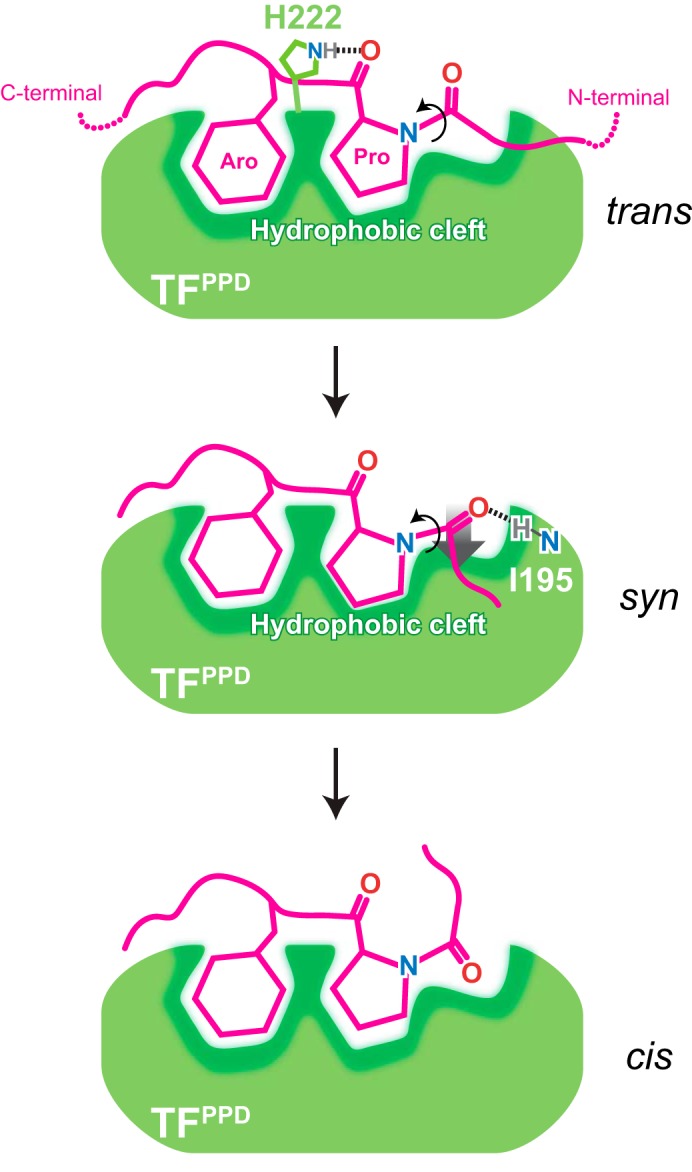
Schematic representation of peptidyl–prolyl cis/trans isomerization by TFPPD. TFPPD and the substrate protein are show in green and magenta, respectively. TFPPD captures the proline-aromatic motif in the trans form located in the hydrophobic stretches of the substrate protein. The interaction is mainly mediated by hydrophobic interactions with the conserved hydrophobic cleft of TFPPD that is decollated by TF His222 forming a hydrogen bond with the backbone carbonyl oxygen of the proline residue in the substrate. When the peptidyl–prolyl bond rotates to syn form, the carbonyl oxygen of the amino acid residue preceding the proline residue forms an intermolecular hydrogen bond with backbone amide group of TFPPD Ile195, which tethers the peptidyl–prolyl bond onto the hydrophobic surface of the TFPPD. Both the intermolecular hydrogen bond and the hydrophobic environment are important for efficient cis/trans isomerization. When the peptidyl–prolyl bond rotates to cis form, the intermolecular hydrogen bond, and consequently the close hydrophobic contact are released.
