Figure 4.
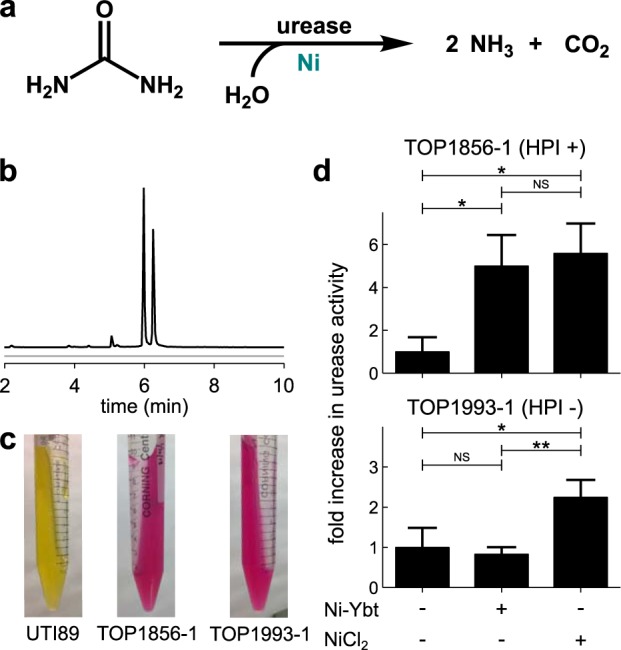
Ni–Ybt import supports urease activity in Klebsiella pneumoniae. a, the Ni-dependent urease enzyme catalyzes hydrolysis of urea, releasing ammonia and carbon dioxide. b, LC-MS analysis of the conditioned media from cultures of the two K. pneumoniae isolates, TOP1856–1 (black) and TOP1993–1 (gray). Only TOP1856–1 produces Ybt. Ion chromatograms are presented with identical scaling. c, TOP1856–1 and TOP1993–1, as compared with UTI89, are positive for urease activity using Christensen's urea agar test (59). d, the K. pneumoniae isolates, TOP1856–1 and TOP1993–1, were grown in a minimal media (W4) optimized for high urease expression (34). Addition of NiCl2 (1 μm) significantly enhanced urease activity of both strains, whereas addition of purified Ni–Ybt (1 μm) only enhanced urease activity for the HPI-positive strain TOP1856–1. Urease activity is shown relative to the unsupplemented condition. Data are shown as mean ± S.D. *, p ≤ 0.05; **, p ≤ 0.01 based on a t test (two-tailed); NS, nonsignificant.
