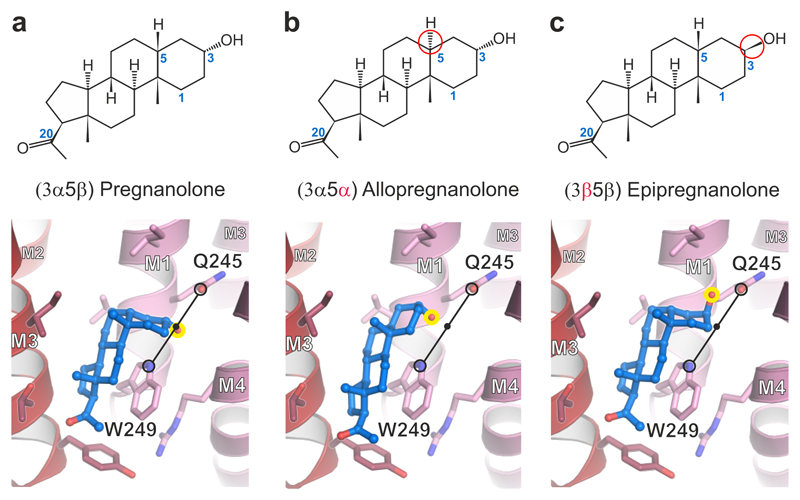
Figure 4.
Computational docking of pregnanolone and related neurosteroids. Structural formulae and α5TMD structures showing binding modes of computationally docked (a) pregnanolone, (b) allopregnanolone and (c) epipregnanolone. Stereoisomeric differences from pregnanolone are indicated in formulae by red circles. In the 3D models, the critical C3 hydroxyl group is highlighted by a yellow circle. Potentiators possess a 3α-hydroxyl which is computationally docked equidistantly between the Gln245 εO Gln and Trp249 εN (3.5 Å for pregnanolone; 3.6 Å for allopregnanolone; indicated by a black line) regardless of whether the rings assume a half-chair or flat geometry. The C3 hydroxyl of 3β pregnanolone can only share a putative hydrogen bond with the Gln245 amide (2.8 Å distance).