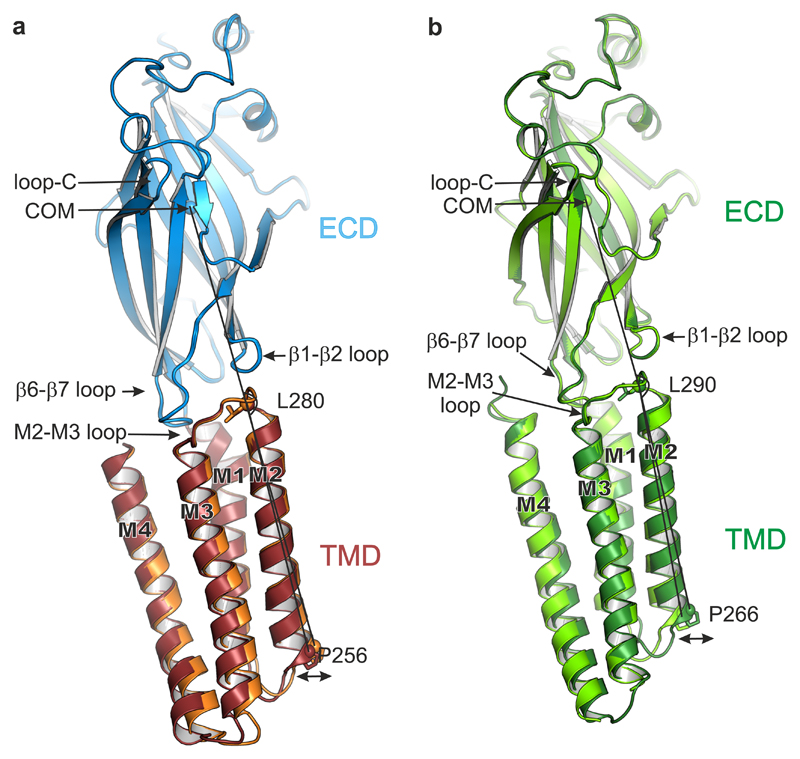
Figure 6.
TMD motions relative to the ECD. (a) Side-on view of a single subunit (B chains), from apo α5TMD (orange TMD) and pregnanolone-bound α5TMD (ruby TMD), superposed by their ECDs (blue). The ECD conformations are very similar (RMSD of 0.19 Å over 204 equivalent Cα positions). However, the lower half of the pregnanolone-bound TMD flexes such that the desensitisation gate at Pro256 swings outwards from the pore (indicated by double-headed arrow). The angles measured between the ECD centre of mass (COM) and the Cα atoms of Leu280 at the top of M2 and Pro256 at the bottom of M2 are 162.5° (apo α5TMD) and 164.4° (pregnanolone-bound α5TMD), respectively, i.e. a 1.9° swing. (b) Equivalent view and superposition between open GlyR (light green, PDB ID: 3JAE) and desensitized (or partially open) GlyR (dark green, PDB ID: 3JAF). The narrowest pore diameter is at -2' (Pro266) position in both cases (8.8 Å and 5 Å, respectively). The GlyR ECD conformations are also very similar (RMSD of 0.31 Å over 210 equivalent Cα positions in chains B). As observed in α5TMD, relative to the superposed ECDs the lower half of the GlyR TMD flexes such that the desensitization gate at Pro266 is displaced outwards from the pore to expand and open the channel. The angles measured between the ECD COM and the Cα atoms of Leu290 at the top of M2 and Pro266 at the bottom of M2 are 161.4° (desensitized GlyR) and 164.1° (open GlyR), respectively, i.e. a 2.7° swing.