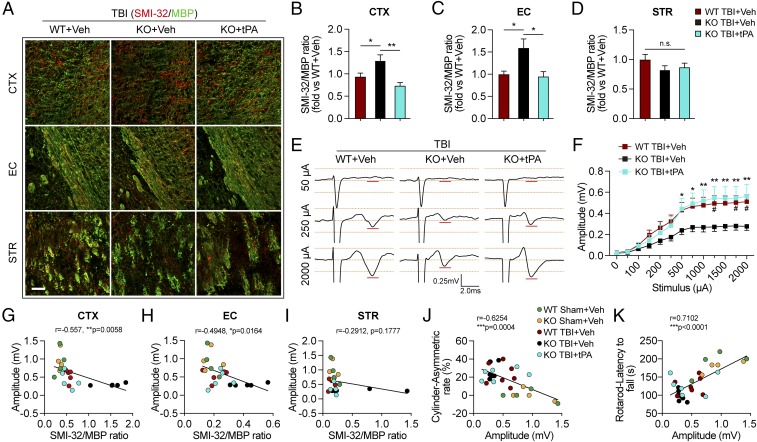
Fig. 5.
Intranasal delivery of recombinant tPA mitigates long-term white matter injury and improves axonal conduction after TBI in tPA-KO mice. WT and tPA-KO mice were subjected to sham injury or unilateral TBI in the right hemisphere. tPA (0.5 mg/kg) or an equivalent volume of vehicle (PBS) was delivered intranasally 2 h after TBI and every other day up to 14 d postinjury. (A) Representative images of SMI-32 (red) and MBP (green) immunostaining 35 d after TBI. (Scale bar, 50 μm.) (B–D) Quantification of SMI-32/MBP intensity ratio in CTX (B), EC (C), and STR (D) after TBI. n = 7–11, normalized to WT+vehicle. (E) Representative traces of evoked CAPs in the ipsilesional CC and EC (stimulus, 50 μA, 250 μA, and 2,000 μA 0.75 mm lateral to the stimulating electrode) at 35 d post-TBI. Recordings were made ventral to the lesion core (Fig. 3F). (F) Action potential conduction along nerve fibers, as measured by the amplitude of the CAPs under different stimulus strengths (0–2,000 μA). (G–I) Correlation analyses of evoked CAPs amplitude (under the stimulus of 2,000 μA) and the ratio of SMI-32/MBP intensity in CTX (G), EC (H), and STR (I). (J and K) The evoked CAP amplitude (under the stimulus of 2,000 μA) was correlated with the forelimb use asymmetry in the cylinder (J) and latency to fall off the rotarod (K). Data are presented as mean ± SEM. n = 7–11 per group in B–D; n = 8–12 per group in E and F; n = 4–7 per group in G–K. *P ≤ 0.05, **P ≤ 0.01 by one-way ANOVA followed by Tukey post hoc correction in B–D; #P ≤ 0.05 WT TBI+vehicle vs. KO TBI+vehicle and *P ≤ 0.05, **P ≤ 0.01 TBI-KO+tPA vs. TBI-KO+vehicle by two-way repeated-measures ANOVA followed by a Tukey post hoc correction in F. P values stated in G–K were determined by two-tailed Pearson product linear correlation analyses. n.s., not significant.