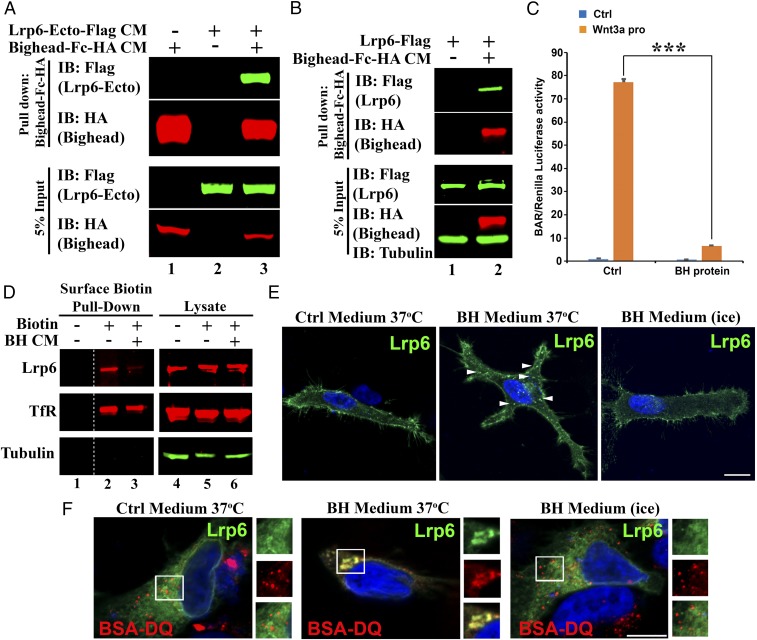
Fig. 5.
Bighead binds to Lrp6 and promotes its endocytosis. (A) Bighead bound to the Lrp6 extracellular domain. CM for a secreted form of LRP6 ectodomain-Flag and Bighead-Fc-HA was allowed to bind as indicated, and subjected to protein A/G agarose pull-down followed by immunoblotting (IB). Total protein expression in the CM was confirmed by IB of the input. (B) Bighead also bound to full-length Lrp6. HEK293T cells transfected with full-length Lrp6-Flag were incubated with control or Bighead-Fc-HA CM for 3 h, and cell lysates were subjected to protein A/G agarose pull-down followed by IB. Total protein expression in the lysate was confirmed by IB of 5% of the input. Tubulin served as a cell lysate loading control. (C) Bighead (BH) protein inhibits Wnt3a protein-induced β-catenin–activated reporter (BAR) reporter expression. HEK293T BAR-Luc/Renilla stably transfected cells were pretreated with or without BH-Fc-HA affinity-purified protein for 6 h, and 100 ng/mL Wnt3a protein was then added to the CM. Cells were further cultured for 16 h, and Luciferase/Renilla activity was measured. The experiment was performed in triplicate, and data are represented as the mean ± SD after normalization to Renilla activity (***P < 0.005). Ctrl, control. (D) BH treatment reduces cell-surface levels of endogenous Lrp6. HEK293T cells were treated with control or BH CM for 1 h at 37 °C, and endogenous cell surface proteins were labeled with sulfo-NHS-SS-Biotin on ice for 30 min. Cell lysates were subjected to pulldown with Streptavidin-agarose beads followed by IB. Total protein expression in the lysate was confirmed by IB of the input. Transferrin Receptor (TfR) was used as a control receptor that is recycled independent of the Wnt pathway. Tubulin served as a loading control. Note that BH reduced cell surface Lrp6, but not TfR (compare lanes 2 and 3). The dashed line indicates noncontiguous lanes. (E) BH induces LRP6 endocytosis. HeLa cells transfected with LRP6-Flag were treated with Ctrl or BH CM for 1 h at 4 °C or 37 °C as indicated, and processed for immunofluorescence. Arrowheads indicate internalized Lrp6+ vesicles. Note that BH induced Lrp6+ vesicles at 37 °C, but not at 4 °C. Another Lrp6 endocytosis experiment is presented in SI Appendix, Fig. S6D. (Scale bar: 20 μm.) (F) BH induces Lrp6 internalization into endolysosomes. HeLa cells transfected with LRP6-Flag were preincubated with BSA-DQ. Cells were then treated with Ctrl or BH CM for 1 h at 37 °C or 4 °C as indicated. Cells were processed for immunofluorescence. (Scale bar: 20 μm.) Squared areas are shown in individual channels as enlarged Insets (1.5× digital enlargement) on the right of each immunofluorescence panel. Note that Lrp6 was endocytosed into lysosomes containing BSA-DQ internalized from the culture medium and that Lrp6 vesicles were eliminated on ice, which prevents endocytosis.