Abstract
The sustainability of cobalt is an important emerging issue because this critical base metal is an essential component of lithium-ion batteries for electric vehicles. More than half the world’s cobalt mine production comes from the Katanga Copperbelt in DR Congo, with a substantial proportion (estimated at 15-20%) being extracted by artisanal miners. Here we show, in a case study performed in the town of Kolwezi, that people living in a neighbourhood that had been transformed into an artisanal cobalt mine, had much higher levels of cobalt in urine and blood than people living in a nearby control area. The differences were most pronounced for children, in whom we also found evidence of exposure-related oxidative DNA damage. It was already known that industrial mining and processing of metals have led to severe environmental pollution in the region. This field study provides novel and robust empirical evidence that the artisanal extraction of cobalt that prevails in the DR Congo may cause toxic harm to vulnerable communities. This strengthens the conclusion that the currently existing cobalt supply chain is not sustainable.
Cobalt is essential for numerous modern applications.1,2 More than 50% of the world’s current production of cobalt goes to rechargeable batteries for smartphones, laptop computers and electric vehicles.3 Because cobalt is an essential element in lithium-ion batteries, the anticipated rising demand of electric vehicles has led to an increase in the market for cobalt and a surge in price, such that cobalt has been dubbed “the hottest commodity of 2017.”4 However, whilst the (generally beneficial) impact of electric vehicles in terms of greenhouse gas emissions has been studied extensively, the sustainability of cobalt has been evaluated mainly with regard to the vulnerability of its supply, rather than in terms of its environmental or human impacts.5,6 Cobalt features in the EU 2017 list of critical raw materials on account of its low substitution and recycling rates, and its supply risk,7 and also in the recently released list of 35 mineral commodities “deemed critical to the economic and national security of the USA”, mainly because of its vulnerability to supply restrictions.8 Cobalt is indeed unique among the base metals in that its supply is dominated by a single country, the Democratic Republic of Congo (DRC), which produces about 60% of worldwide cobalt, with no other country producing more than 6%.3,9 However, the DRC is one of the poorest countries in the world and it sits in the lowest decile of countries with regard to World Governance Indicators and Environmental Performance Index.10,11,12
In the DRC, cobalt is mined in the Katanga Copperbelt, an area that contains some of the richest cobalt deposits in the world.13 The global increase in demand of cobalt has led, since about 2000, to a boom in mining of cobalt in Katanga.9 In 2009, we documented high exposure to cobalt and other trace elements in people living within 3 km of industrial mines or smelting operations.14 However, in Katanga, cobalt is also extracted in small artisanal mines, because it is abundant in surface deposits, mainly as heterogenite, a mixed oxide and hydroxide of cobalt (CoOOH), that is often concentrated in thin and friable layers.15 This has led to widespread artisanal mining, with thousands of “creuseurs” (diggers) extracting heterogenite in precarious and hazardous conditions.16,17,18,19 The mineral is bought by traders, who sell it to Chinese, Indian or Lebanese companies, for further export to cobalt-refining countries (China, Belgium, Finland and Canada).3,20 The share of cobalt produced by artisanal mining is fluctuating and difficult to determine because of its informal and often illegal character.3 In 2015-16, 12,000-18,000 tons, i.e. 15-20% of the DRC’s total cobalt production, were estimated to come from artisanal mine sites.20
Nongovernmental organizations18 and the media21 have denounced the human rights abuses accompanying the artisanal extraction of cobalt in Katanga, but little scientific research has been devoted to the health implications for people living in the vicinity of artisanal cobalt mines. Here, we provide robust quantitative exposure data demonstrating the possible human health costs in this early phase of the cobalt supply chain.
Case study
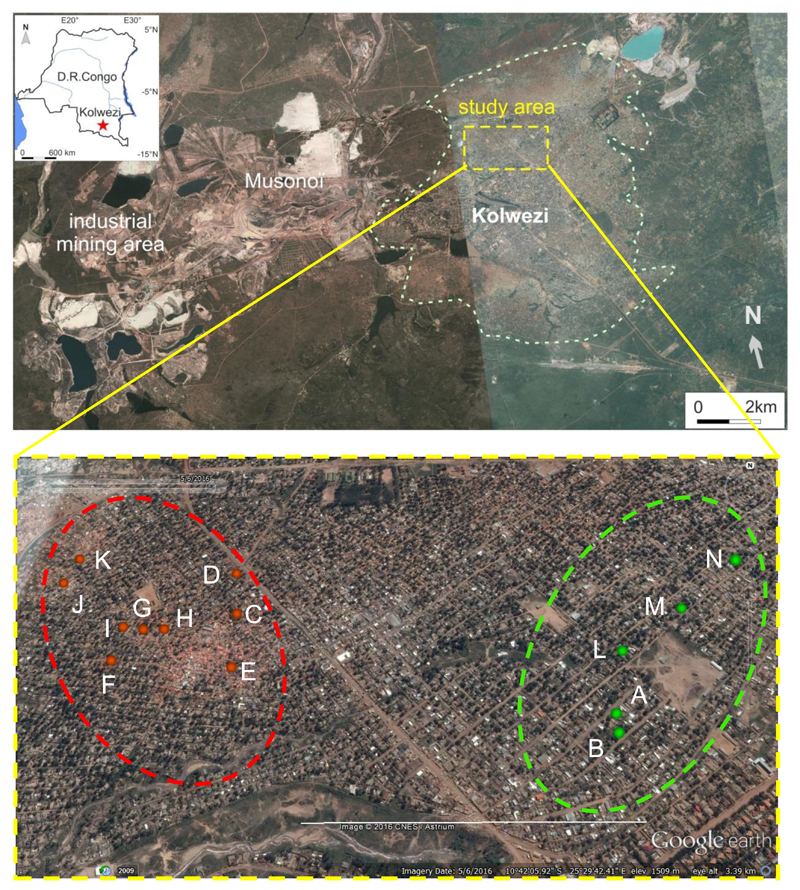
Our study took place in Kolwezi, a city of about 450,000 inhabitants and an important mining centre since the first half of the 20th century, with large open-pit mines located to the west of the city (Figure 1). The study was initiated because of concern – voiced by local authorities, civil society and non-governmental organizations – about the health risks for people living in Kasulo, a densely populated urban neighbourhood where artisanal mining had started, early in 2014, after a resident had discovered, reportedly while digging a pit latrine, that his house was located on a cobalt-rich substrate. Within a few months, numerous properties of the neighbourhood contained one or more mine pits (leading to underground mineshafts) dug by hundreds of “creuseurs”, and piles of mine tailings covered large portions of the surface of most plots (Figure 1 and Supplementary Figure 1). Most residents continued living in their homes and many participated in one way or another in the lucrative activities that had arisen from the new bonanza.
Figure 1. Satellite images of Kolwezi and the study area.
Upper panel: satellite image (Google Earth) of the Kolwezi area with its urban residential zone (within white dashed line) and the zone of industrial mining to the West of the town. The study area (within yellow dashed line) is shown in detail in the lower panel.
Lower panel: study area showing the chosen control area without mining activities (on the right circled by a green dashed line) and the approximate area affected by artisanal mining (on the left circled by a red dashed line). The red and green dots with letters A to N indicate the 14 plots where participants were recruited. Zones of reddish coloration within the mining area are due to orange plastic sheeting used for sheltering or covering mine pits (Imagery date is 5/6/2016). The white horizontal line corresponds to 1000 m.
The main purpose of our investigation was to assess, via biomonitoring, the residents’ and mineworkers’ internal exposure to cobalt and toxic trace elements associated with the ore, especially uranium. We conducted two brief campaigns, in November 2014 and May 2015, to collect environmental and human samples in the new mining site, and in a nearby control area without current or past mining.
Environmental assessment of the studied area
The concentrations of aqua regia extractable metals in ore samples and surface dust are presented in Supplementary Table 1. In the ore, the average concentration of cobalt amounted to about 26,000 µg/g (2.6%). Other abundant trace elements were copper and manganese (both around 1,500 µg/g), nickel and vanadium (both around 200 µg/g) and uranium (44 µg/g). These concentrations fit with the known elemental composition of heterogenite, the main cobalt-bearing mineral in the region.15
Supplementary Figure 1 illustrates how various processes contributed to contamination of the environment in the new mining area: spillage of ore from bags hoisted from the pits, handling and ore stockpiling on the premises and inside houses, in-situ crushing of ore blocks and handpicking ore fragments, accumulation of mine tailings around the houses. These activities contributed further to the dustiness that typically prevails in environments with few hardened surfaces, thus leading to substantial accumulation (and continuous resuspension) of ore-contaminated dust, not only outdoors on roofs, yards and unpaved paths or streets, but also indoors on dirt floors, furniture, kitchenware, food items, clothes, toys and other objects. In the nearby control area, the general physical environment (density and types of dwellings, dirt floors, and unpaved roads) was similar to that of the mining area, except for the absence of mining activities.
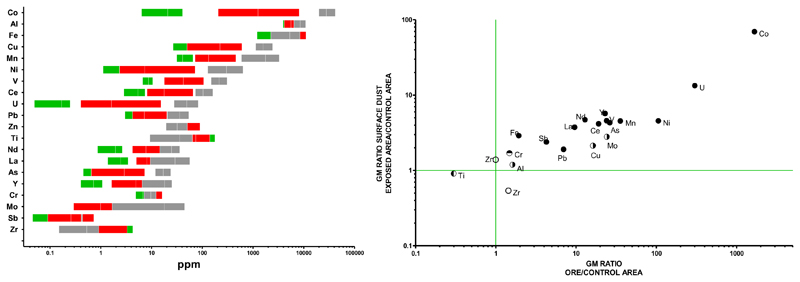
The composition of trace elements in outdoor and indoor surface dust collected in the residential area affected by mining corresponded closely to that in the ore, including for accessory elements (e.g. the lanthanides), thus confirming enrichment by the mined ore (Figure 2). On average, surface dust contained 1,100 µg/g cobalt (70-fold higher than in the control area) and 2 µg/g uranium (13-fold higher than control), i.e. a Co:U weight ratio of 550:1 comparable to that in the ore (590:1). Cobalt in surface dust from the exposed area largely exceeded the (Canadian) standard of 22 µg/g for surface soil for residential property use.22 Standards were not exceeded for other elements, except for copper (average of 193 µg/g; standard of 140 µg/g 22).
Figure 2. Concentrations of trace elements in surface dust and ore.
Left panel: concentrations of trace elements, ranked by their abundance in 3 samples of ore (grey bars), surface dust from the mining (exposed) area (9 plots, red bars) and the control area (5 plots, green bars); bars represent the range of values with mean (for some elements, green bars are partially or totally hidden behind another bar).
Right panel: Ratio of geometric mean (GM) concentrations of metals in surface dust from the exposed area over the control area (y-axis) against ratio of GM concentrations of metals in ore over control surface dust (x-axis). Confidence intervals of GM ratios can be found in Supplementary Table 1. Full symbols indicate both GM ratios are significantly higher than 1, half-filled symbols indicate GM ratio differs significantly from 1 only for ore/control (symbol divided horizontally) or only for exposed/control (symbol divided vertically). Spearman correlation coefficient for the 20 data points is 0.85 (95% CI 0.65–0.94, p<0.0001)]
The metal concentrations in samples of drinking water provided by the participants did not differ significantly between the mining and control area (Supplementary Table 2), and no values exceeded WHO standards.23
Biomonitoring data
In the area affected by mining, we recruited 72 residents (40 adults, 32 children) living in nine plots, and 25 mineworkers; in the control area we recruited 25 residents (12 adults, 13 children) living in five plots (Table 1 with additional details in the Methods section).
Table 1. Demographic characteristics of the population.
| Residents | Diggers | ||
|---|---|---|---|
| Control area | Mining area | ||
| Number of subjects included | 25 | 72 | 25 |
| November 2014/May 2015 | 10/15 | 43/29 | 15/10 |
| Number of adults (≥14 y) | 12 | 40 | 25 |
| Male/Female | 3/9 | 12/28 | 25/0 |
| Age (y) mean (SD) | 36.8 (15.9) | 37.2 (17.9) | 31.8 (6.0) |
| range | 16–57 | 14–72 | 21–45 |
| Smokers (all men) | 0 | 3 | 18 |
| Number of children (<14 y) | 13 | 32 | |
| Male/Female | 3/10 | 19/13 | |
| Age (y) mean (SD) | 8.5 (3.1) | 6.2 (2.9) | |
| range | 3–13 | 1–11 | |
Values for age are means with standard deviations (SD)
Complete and detailed biomonitoring results are reported in Supplementary Tables 3 to 6. Henceforth, we will concentrate on the most relevant elements, i.e., cobalt, uranium and manganese.
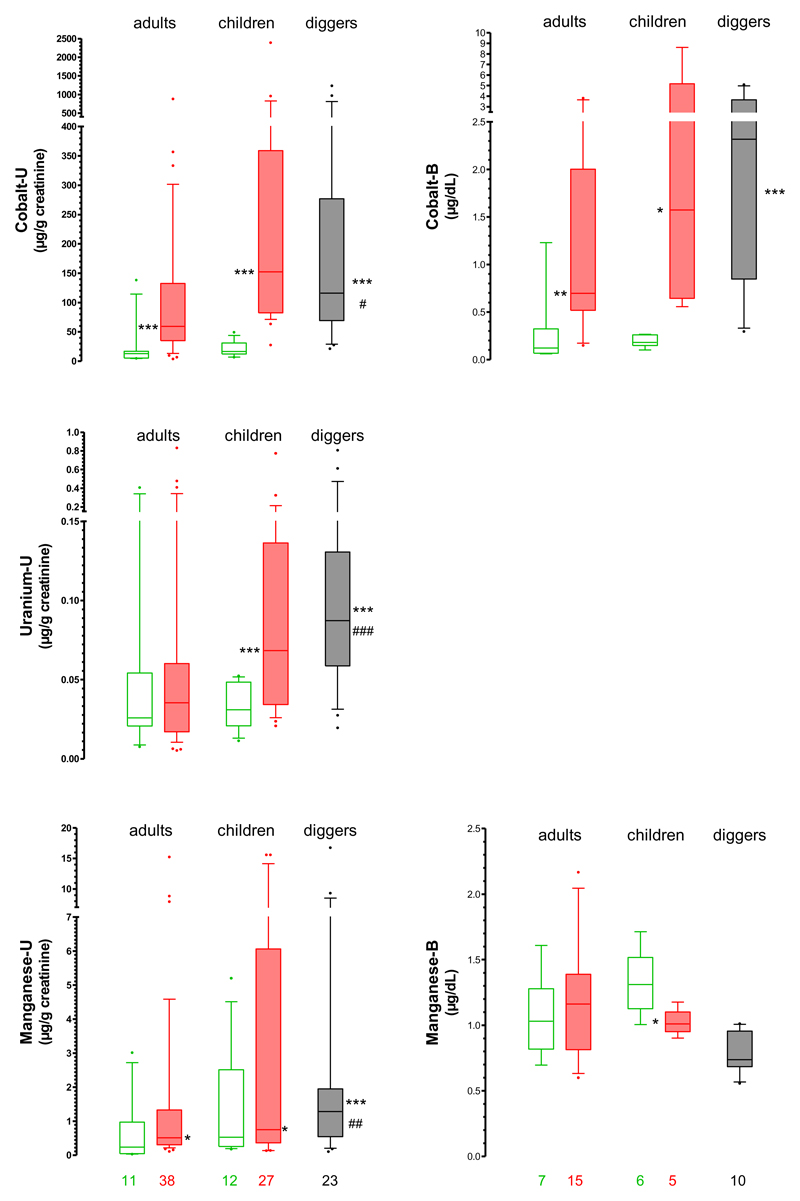
In urine, geometric mean (GM) concentrations (µg/g creatinine) of cobalt, manganese and uranium were higher among exposed than control residents, with and without adjustment for sex and age (Supplementary Table 3). Cobalt exhibited the highest contrast between the two groups [GM ratio 7.1; 95% confidence interval (CI) 4.3-11.6]. Manganese was more than two-fold higher (GM ratio 2.4; 95%CI 1.3-4.5) and uranium almost two-fold higher (GM ratio 1.7; 95%CI 1.0-2.8) among exposed residents. Adjustments for plots did not substantially modify these associations, but led to a loss of statistical significance for uranium.
Stratification by age category revealed more pronounced differences for children than for adults (Figure 3, Supplementary Table 4). Urinary concentrations of cobalt were 9.3-fold (95%CI 4.7-18.4) and 5.2-fold (95%CI 2.4-10.9) higher among exposed children and adults than among control children and adults, respectively. Urinary uranium did not differ significantly between exposed and control adult residents, but exposed children had twice (95%CI 1.2-4.0) as much uranium in urine than control children. Urinary manganese was more than two-fold higher in exposed adults (95%CI 1.1-5.4) and children (95%CI 1.2-6.3) than in corresponding controls.
Figure 3. Concentrations of cobalt, uranium and manganese in urine (left), and of cobalt and manganese in blood (right).
Data from residents in the control area are shown in green (open); data from residents in the mining area are shown in red (filled); data from mineworkers (diggers) are shown in black. Uranium was below detection limits in blood. Box plots with medians and 25th-75th percentiles, and 10th-90th percentiles for whiskers; numbers of subjects in each group are indicated at bottom of graph. * p<0.05, ** p<0.01, *** p<0.001 for comparison with control subjects (adults or children, as appropriate); # p<0.05, ## p<0.01 for comparison with exposed adult residents (see Supplementary Tables 4 and 6 for details).
The group of diggers exhibited substantially (2 to 11-fold) higher urinary cobalt, uranium and manganese, than adult residents from either the control or exposed groups (Figure 3).
In the second campaign, we also took blood samples, because we were concerned that the high metal concentrations in urine samples from the first campaign could have been due to external contamination by dirty hands or clothing. However, the latter possibility can be reasonably ruled out, since blood concentrations of cobalt were markedly higher in exposed adults [age- and sex-adjusted GM ratio 5.7 (95%CI 2.0-15.7)], exposed children [GM ratio 7.5 (95%CI 1.5-36.0)] and diggers [GM ratio 13.1 (95%CI 3.3-53.1)] than in corresponding controls (Supplementary Tables 5 and 6, Figure 3). Moreover, the correlation between urinary and blood concentrations of cobalt was strong [Spearman’s rho=0.91 (95%CI 0.84-0.95)] (Supplementary Figure 2). Levels of uranium in blood were below the detection limit for all subjects. Blood levels of manganese did not differ between adult residents, but they were lower in exposed children than in control children [GM ratio 0.7 (95% CI 0.5-0.9)]; the diggers also had lower blood manganese concentrations than the other adults (Figure 3).
Table 2 presents the associations (adjusted for age and sex) between concentrations of cobalt, uranium or manganese in urine or blood in individual subjects, and concentrations of these metals in surface dust in their home environment. We found strong associations for cobalt in both adults and children, and for both urine and blood. Among children, significant positive associations were also found for uranium and manganese in urine, but an inverse association was found for manganese in blood. No significant correlations were found between biomonitoring data and metal concentrations in drinking water (not shown).
Table 2. Adjusted associations, expressed as β coefficients, between cobalt, uranium or manganese concentrations in urine or blood and in surface dust among residents.
| ALL SUBJECTS | ADULTS | CHILDREN | |
|---|---|---|---|
| URINE | N=88 | N=49 | N=39 |
| ln(Co-U)i against ln(Co-dust)plot |
0.37 *** (0.21–0.52) |
0.27 ** (0.07–0.47) |
0.46 *** (0.29–0.62) |
| ln(U-U)i against ln(U-dust)plot | 0.18 (-0.09–0.45) |
0.03 (-0.29–0.35) |
0.29 * (0.02–0.56) |
| ln(Mn-U)i against ln(Mn-dust)plot |
0.43 * (0.08–0.78) |
0.35 (-0.15–0.86) |
0.54 * (0.03–1.06) |
| BLOOD | N=33 | N=22 | N=11 |
| ln(Co-B)i against ln(Co-dust)plot |
0.46 *** (0.30–0.61) |
0.38 *** (0.15–0.60) |
0.57 *** (0.41–0.74) |
| ln(Mn-B)i against ln(Mn-dust)plot | 0.01 (-0.13–0.15) |
0.02 (-0.13–0.17) |
-0.40 ** (-0.68–-0.12) |
Associations, expressed as β coefficients (with 95% confidence intervals) of the regressions, adjusted for sex and age, between individual (i) natural-log (ln) concentrations of Co, U or Mn in urine (-U) or blood (-B) and natural-log concentrations of Co, U or Mn in surface dust sampled at their residence (14 plots). Significant associations are shown in bold: *** p<0.001, ** p<0.01, * p<0.05. Italics indicate a negative association
Oxidative DNA damage
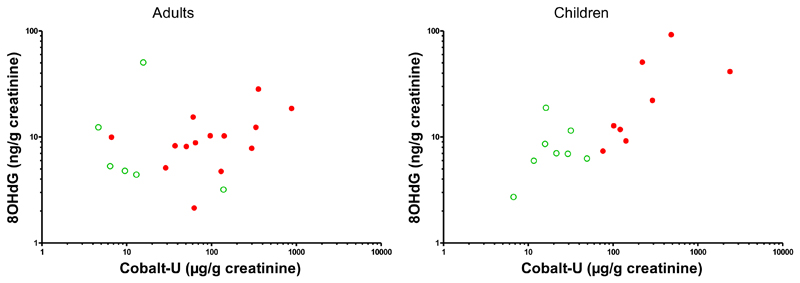
Urinary concentrations (ng/g creatinine) of 8-hydroxydeoxyguanosine (8OHdG), an index reflecting oxidative DNA damage, did not differ significantly (p=0.16) between exposed residents (GM 14.2, 95%CI 7.4-27.5, n=22) and control residents (GM 6.8, 95%CI 3.8-12.9, n=14). However, after stratification by age group, 8OHdG levels were much higher (GM ratio 6.7, 95%CI 1.9-23.4, p=0.006) in exposed children (GM 45.0, 95%CI 21.2-95.6, n=8) than in control children (GM 7.9, 95%CI 4.0-15.9, n=8), whereas this was not so (GM ratio 1.4, 95%CI 0.4-5.2, p=0.57) when comparing exposed adults (GM 7.4, 95%CI 3.4-16.1, n=14) with control adults (GM 5.6, 95%CI 2.1-15.2, n=6). The concentrations of 8OHdG correlated with those of cobalt in urine among children (p<0.001), but not among adults (Figure 4).
Figure 4. Relation between concentrations of cobalt and 8-hydroxydeoxyguanosine (8OHdG) in urine.
Individual data from adult residents (left panel) and children (right panel). Data from residents in the control area in green open symbols, data from residents in the mining area in red filled symbols. Note logarithmic scale of x-axis and y-axis. Spearman correlation is nonsignificant among adults (rho=0.23; 95%CI -0.25–0.62) and highly significant among children (rho=0.78; 95%CI 0.46–0.92; p<0.001).
Discussion
Our observations provide unprecedented scientific documentation of how the (unregulated) extraction of a strategic metal commodity may rapidly degrade the local environment and lead poor communities to becoming exposed to toxic hazards.
We previously documented high exposure to cobalt and other trace elements in people living close (<3 km) to industrial mining or smelting operations.14 Here, we compared residents of a well-defined urban neighbourhood that had recently been transformed into an artisanal mine, with residents of an appropriate control area. Concurrent biomonitoring among artisanal mineworkers allowed direct comparisons between residents and workers. We demonstrated highly significant correlations between the degree of cobalt enrichment of surface dust and cobalt levels in urine and blood of adult and children residents (Table 2), thus strongly indicating that dust exposure (rather than, e.g., contamination of drinking water) was the dominant pathway for the excessive intake of cobalt. Finally, we not only documented excessive trace metal exposure, we also provided evidence of oxidative stress and DNA damage (among exposed children).
The adverse environmental and human impacts of small-scale and artisanal mining have been studied almost exclusively for gold mining, usually with a focus on the use of mercury,24 but also in relation to lead.25 Our study is unique in that it concerns cobalt, a critical base metal that is essential for modern technology, most notably rechargeable lithium-ion batteries for electric vehicles.1–3
The main finding of our biomonitoring study is that the residents of Kasulo, and especially the children, were heavily contaminated by cobalt. To put the obtained figures in perspective, the average concentrations of urinary cobalt in the adults (64 µg/g creatinine), children (193 µg/g creatinine) and miners (133 µg/g creatinine) largely exceeded 15 µg/L, the level not to be exceeded in the workplace according to the American Conference of Governmental Industrial Hygienists.26 Biomonitoring studies in workers from various industries have shown good correlations between cobalt levels in urine or blood and recent exposure to cobalt.27 Cobalt concentrations in blood and urine similar to those found in the present study have been reported for cobalt refinery workers in the early 1990s.28
Besides high internal levels of cobalt, we also found evidence, among children, of a high urinary excretion of uranium and manganese, i.e. metals associated with the ore.
Exposure assessment
Even among our control subjects, many trace elements were elevated in urine or blood when compared with reference values derived from population surveys in high income countries,29,30,31 and, in the case of cobalt, even when compared with occupational standards.26 These high control values can be explained by the pollution caused in and around Kolwezi during decades of industrial copper and cobalt mining, with little or no concern for the environment. The western part of the town is bordered by a wasteland of disused mines and tailings, where the ore is readily accessible and may generate metal-rich dust in the dry season.32 These high background values illustrate the importance of selecting appropriate comparison populations to assess over-exposures.
Although child labour has been reported in artisanal mines in Katanga,18,33 the high biomonitoring values of cobalt found among children from the mining area were not obtained from children engaged in work. The propensity of children to be more heavily exposed to environmental pollutants than adults is a well-established phenomenon,34 that has been mainly documented for lead,25 and that we have also observed in our previous studies.14,35 The reasons for the higher internal exposure of children may be physiological (high gastrointestinal absorption) and behavioural, such as frequent hand-to-mouth contact and playing close to the ground. In an unpublished study performed in Lubumbashi, we found much higher quantities of ingested dust (based on tracer metals in faeces) in children (median 0.5 g/day) than in adults from the same households (median 0.07 g/day), these quantities being considerably higher than default values for daily soil and dust ingestion determined for industrially developed countries (0.1 and 0.05 g/day, respectively).36
The high urinary concentrations of uranium in exposed children and miners suggest the uranium present in the ore is bioavailable, at least to some extent. We attempted to measure uranium in blood, but all values proved to be below the detection limit. Correlations have been found between levels of uranium in urine and drinking water,37 and increased biomonitoring values of uranium have been reported in people living close to uranium deposits.38,39
The relations between manganese in urine and in dust were qualitatively similar to those observed for cobalt. However, blood manganese did not correlate positively with manganese in urine or in dust, since children in the mining site had lower blood manganese than control children, and mineworkers had the lowest blood levels of manganese. The kinetic behaviour of manganese is complex and the literature on the biomonitoring of manganese is inconsistent.40 Studies are needed to understand the toxicokinetics of manganese, especially with co-exposure to other metals.
Limitations
The cross-sectional design of our study is a limitation but there is little doubt that the high levels of cobalt observed in the residents of the mining site resulted from the ongoing local mining activities.
One could criticize the small numbers of participants in our study. The logistic and other obstacles encountered to perform such a seemingly modest field survey should not be underestimated. Regardless, low power is of concern mainly in the absence of significant results, whereas the highly significant differences found here are indicative of strong and consistent effects. Nevertheless, we cannot claim that our participants were perfectly representative of the exposed and control populations. However, we do not think that our non-random, “convenience” sampling of participants seriously biased our results: in the mining area, we did not recruit the worst affected properties and in the control area, we did not select the cleanest looking houses. Small sample sizes, however, render studies vulnerable to misclassification. Thus, as reported in the Methods section, some control participants were possibly exposed indirectly to mining-related dust, and although the participating residents of the mining area were not mineworkers, some reported working sometimes as diggers or had a household member who was a digger, and many residents, including children, occasionally handled bags of ore or sorted minerals. Consequently, these casual occupational and para-occupational exposures may have somewhat exaggerated the “purely residential” exposure of the people living in the mining area.
A weakness of our study is our inability to present reliable figures for the size of the population that was potentially impacted by the mining activities, but we estimate (based on the number of dwellings) that at least 5,000 people lived in the affected area. Official figures of the number of workers involved, let alone the amount of ore production, are also lacking for the studied site. Such paucity of data is symptomatic of the weak governance prevailing in the local mining sector.
Health significance
Our primary purpose was to assess exposure, not to evaluate the health impact in the mining-affected community, which would have required a different approach and more resources. Nevertheless, we found that (creatinine-corrected) urinary 8OHdG was higher among the children in the mining site than among the control children, indicating that the former had undergone more oxidative DNA damage than the latter.41 Some caution is warranted because assaying 8OHdG by ELISA is more variable than by other quantification methods,42 but high variability is unlikely to be responsible for the almost 7-fold difference observed between exposed and non-exposed children. That urinary 8OHdG did not differ significantly between exposed and control adults, may be due to the higher contrast in internal exposure among children than among adults, but it is also compatible with the notion that children are more susceptible to environmental pollutants.34 The correlation between 8OHdG and cobalt in urine does not necessarily imply that cobalt was the main culprit for the oxidative injury. In fact, 8OHdG levels were also associated with other elements, possibly indicating that the oxidative damage resulted from the mixture of metals (or other factors associated with the exposure), rather than any specific metal.
What long-term morbidity could result from the high exposure to the trace metals in mine dust? High doses of cobalt may affect the heart, lungs, blood and thyroid.27,43 Manganese is mainly neurotoxic.44 Uranium is mainly nephrotoxic, but it could also be neurotoxic.45 Exposure to gaseous radon, one of uranium’s radioactive decay products, must also be considered, especially for the diggers, who work in poorly ventilated mineshafts, and for the occupants of homes where minerals may be stored for prolonged times. Therefore, epidemiological studies of the health consequences should cover a broad range of endpoints such as birth defects, neurodevelopmental impairment, respiratory disorders, heart and kidney disease, and cancer. The evidence of increased oxidative DNA damage found among the highly exposed children suggests a higher occurrence of genetic and epigenetic changes and, hence, points to an increased risk of cancer in later life.
Ethical considerations and societal implications
Individual results were given to the local health workers for communicating them to the participants, but we recognize that simply telling people they are being poisoned does not help them much. Our overall findings were communicated to the authorities of Kolwezi. Although mining in Kasulo had been officially forbidden, even before our surveys, the situation continued virtually unchanged until mid-2017, when the remaining inhabitants were forcibly relocated after the site had been sold to Congo DongFang Mining (CDM). This led to demonstrations and complaints about the low indemnities paid by CDM.
Environmental degradation and toxic exposures constitute only part of the adverse consequences of the “urban mining” that befell the Kasulo community. The presence of hundreds of diggers and ancillary workers was accompanied by noticeable social disruption, high consumption of alcohol and drugs, prostitution and fights. Nevertheless, the purpose of our article is not to stigmatize the artisanal mineworkers, nor to blame the local residents for the transformation of their own neighbourhood into an unliveable environment. One could object that the Kasulo disaster is not representative of the general situation of artisanal mining in Katanga. Our case study is indeed likely to be a worst case. However, the very occurrence of such extreme conditions is indicative of poor governance, on the one hand, and disregard for sustainability by the buyers of the extracted mineral, on the other. Moreover, qualitatively similar conditions occur in many other locations, where people live or settle close to extractions sites.16,33 The social and economic aspects of artisanal mining in the Katanga copper-cobalt belt have begun to be thoroughly investigated on a larger scale.46
The significance of our study in terms of sustainability hinges on the amount of artisanal mining in the Congo. The proportion of artisanally mined cobalt has been estimated at 15-20% of the total cobalt mined in the DRC.3,20 However, the exact proportion is difficult to establish, partly because ores from artisanal origin are processed together with ores from industrial origin, and because some industrial operators tolerate (or even encourage) exploitation of their mines by artisanal mineworkers, thus further blurring the traceability of the cobalt.20 Moreover, the present case study does not imply that large-scale mining of cobalt, as it is currently taking place in Congo, is more sustainable than artisanal mining. Studies done by us14 and others47 show that industrial operations also lead to high environmental pollution. In other words, a systematic comparative evaluation of the environmental and societal impact of artisanal mining and large-scale mining in Katanga needs to be done. This should include, among other issues, the diversion of young adults from agriculture, thus contributing to food insecurity. Such studies should take into account the perception and needs of the affected populations and the mineworkers themselves.
We acknowledge that our study does not propose solutions to the sustainability problems at stake, but we trust that our findings will provide further incentive for addressing the local and general issues. The relationships between mining, development and the environment are complex.48 This is particularly true with regard to the informal mining sector in the DRC, where artisanal mining provides direct livelihood to approximately 1.2 million “creuseurs”, which implies that some 10 million people indirectly benefit from this activity.49 Artisanal mining constitutes the second largest employment sector in the DRC, after agriculture.50 The currently accepted view is that formalization of the artisanal sector should depart from a legalist viewpoint (“miners must hold valid mining permits”) and focus, using a bottom-up approach, onto the workforce, its livelihood, working conditions, and the practical arrangements that are made among the workers, and between the workers and other stakeholders in the artisanal mining sector.51,52 International regulations such as the U.S. Dodd-Frank Act have focused on ‘conflict minerals’ in Eastern DRC (tin, tantalum, tungsten, gold), whilst leaving the supply chain of non-conflict minerals (copper, cobalt, uranium) largely unregulated.53
The future of mining in DRC largely depends on priorities that lie beyond the sector itself. For artisanal mining, these priorities include bottom-up formalisation; more transparent upstream (miners, traders) trade chain; extensive state reform and the creation of competent and corruption-free state agencies in charge of mining, health and the environment.50,54 These are prerequisite conditions for a sustainable cobalt to produce our batteries.
Methods
Recruitment of participants
The study had a cross-sectional design with data being obtained during two brief campaigns conducted in November 2014 and May 2015. As in our previous field studies,14,35 adults and children (defined as younger than 14 years) were recruited by convenience sampling, with the sampling units consisting of “parcelles” (further called “plots”), i.e. small patches of land containing one or more dwellings (housing one or more families) surrounded by a yard.
After having obtained authorizations from the administrative authority of the city of Kolwezi and then from the head (“chef de quartier”) of the Kasulo district, we went to the target areas, together with one or more community leaders, to approach presumably representative families for participation in the study. After having explained the purpose of the study, we invited an adult man or woman present in the “parcelle” to participate in the survey with other members of the (extended) family, including children. In each plot we intended to include adults and children, trying (informally) to achieve equal numbers of males and females. Refusals were extremely rare and, for reasons of time and logistics, we had in fact to refuse many people who wanted to be included like their neighbours. In the mining area, we also asked mineworkers (all males) who happened to be around, if they wanted to participate. Most of them had worked, as diggers, in pits inside or close to the selected plots on the day of their inclusion.
Subjects gave oral consent to participate for themselves and their children. The study protocol (including the oral consent procedure) was approved by the Committee of Medical Ethics of the UNILU.
We thus included 122 persons: 72 residents and 25 diggers from 9 plots (labelled C to K) in the mining area, and 25 residents from 5 plots (A, B, L, M, N) in the control area (shown in Figure 1, based on GPS coordinates). The sequence of inclusions was as follows:
-
-
November 10th, 2014: plots A and B; plots C and D + 12 diggers
-
-
November 11th, 2014: plots E and F + 3 diggers
-
-
May 14th, 2015: plots G and H + 6 diggers
-
-
May 15th, 2015: plots I, J and K + 4 diggers; plots L, M and N
The number of subjects included per plot was lower in the control area (median 5 subjects, range 4-6) than in the exposed area (median 7 subjects, range 2-25), partly because in the latter area many subjects had insisted on participating in the study; moreover, some plots contained more than one household (with related or unrelated families), thus leading to a plot with 25 participants (plot F). On the other hand, because plot K contained only two participants, we merged this plot with the nearby plot J when adjusting for plots in the statistical analyses.
Characteristics of the participants
The main demographic characteristics of the participants are presented in Table 1. Among adult residents, women were overrepresented, because men were often not at home during daytime; among children there were as many boys as girls, but the sex distributions differed between exposed and control groups, for no obvious reason. The participants from the two areas did not differ by age; nevertheless all statistical comparisons have been adjusted for age and sex. Smoking was rare among residents (3 adult men in the exposed group), but frequent among diggers (18/25 smokers).
Potential misclassification
One woman living in the control area was the wife of a technician employed in an industrial mine company; her high biomonitoring values for cobalt and uranium proved to be outliers among the control subjects, but her data were not excluded. People living in the control area were not restricted to their location and it is likely that they also came close to the mining area, especially along the commercial road separating the two neighbourhoods. Wind-blown dust could conceivably contaminate the control area, but this would tend to decrease the contrast between exposed and control subjects.
On the other hand, residents in the exposed area were not all entirely free from occupational exposures to mine dust. Two participants reported being pit supervisors, two men reported working sometimes as diggers (one of them had worked on the day of sampling, but his urinary results were de facto excluded because of a too high creatinine; he did not have blood results). Some participants had a household member working as a digger (two women with very high values of cobalt and uranium were in this case). In addition, some residents, including children, occasionally or regularly handled bags of ore or sorted minerals.
Survey procedures
The field studies included the following procedures.
Questionnaire. Demographic data (sometimes only an approximate date of birth), information about current and past residence, occupation, smoking, alcohol consumption, medication use, and current or past illnesses were obtained by means of a one-page ad hoc questionnaire that was administered face-to-face in Swahili.
Blood pressure. At the end of the interview and with the subject having remained seated, arterial blood pressure was measured, as a service, using a digital monitor (Omron Healthcare, Hoofddorp, The Netherlands) in all subjects, except in small children. (No differences in blood pressure were found between the groups).
Urine and blood sampling. All subjects gave a spot sample of urine, which was voided directly into 40 ml polystyrene vials with screw caps (Plastiques-Gosselin, Hazebrouck, France). It should be realized that our survey was not done in a clean clinical environment but in challenging field circumstances with precarious toilet facilities. Even though participants were asked to avoid contaminating the urine by their hands, we could not exclude the possibility of contamination by dust particles coming off dirty fingers or clothing during urine voiding or when opening or closing collecting vessels. Consequently, in the second campaign we decided to obtain also a blood sample from most subjects (except small children). An experienced nurse drew a blood sample from a brachial vein into a 4 ml BD Vacutainer® tube with spray-coated K2EDTA (BD367844), after thorough cleaning and disinfecting the skin with alcohol, thus confidently avoiding external contamination of the blood sample.
Water. From all plots, except plots I and J, we obtained a sample of drinking water. We enquired where the family got its drinking water from, and asked one of the adults of the household to pour some drinking water in the same type of polystyrene vessel as used for urine. In both the exposed and control areas, people reported fetching water from municipal (“REGIDESO”) water taps at some distance from their homes. Some households additionally used water from local wells (however, not within the mining area) or rainwater, and some mentioned using (though not drinking) water pumped from the mines. We did not systematically ascertain how drinking water was stored in the house but water was generally kept in closed plastic jerry cans. Water samples were not acidified.
Soil-Dust. In all plots, we collected superficial soil from the yard in front of the house and dust from the floor of its main room (generally a dirt floor), by sweeping an area of about 1 m2 with a household brush into a plastic dustpan, and then placing the collected material into polyethylene minigrip bags. We did not collect deeper soil samples, because there were no (longer) kitchen gardens in the mining area and, hence, no risk of consuming contaminated home grown vegetables. We obtained three samples of the locally mined ore.
GPS coordinates were taken in each plot using a handheld device (eTrex 10, Garmin).
Photographs were taken of people (full-face only with permission) and the surroundings.
Sample treatment and laboratory analyses
All biological samples were kept refrigerated as much as possible, but electrical power was not always available and travel from Kolwezi to Lubumbashi lasted several hours.
Urine and water samples were aliquoted into 4 mL cryovials within two days of sampling. These urine samples, the blood samples and the water samples were kept in a freezer in Lubumbashi until they were transported inside coolboxes as checked luggage, to Belgium, where they were kept refrigerated or frozen until analysis. Dust samples were sieved (2 mm) and crushed (mortar and pestle) in the laboratory of CBLN in Lubumbashi, and then also transported to Belgium for analysis.
The human samples were analysed in the laboratory of the Louvain centre for Toxicology and Applied Pharmacology (Université catholique de Louvain, Belgium) without knowledge of their exact provenance (blind analysis). In urine, 24 elements were quantified as described previously,14,30 using an Agilent 7500ce instrument (Agilent Technologies, Santa Clara, CA, USA). Briefly, urine specimens (500 µl) were diluted quantitatively (1+9) with a HNO3 1%, HCl 0.5% solution containing Sc, Ge, Rh and Ir as internal standards. Li, Be, Al, Mo, Cd, In, Sn, Sb, Te, Ba, Pt, Tl, Pb, Bi and U were analysed using no-gas mode, whilst helium mode was selected to quantify V, Cr, Mn, Co, Ni, Cu, Zn, As and Se. In blood, eight elements (Mn, Co, Pd, Cd, Hg, Tl, Pb and U) were quantified using an Agilent 7500cx instrument after dilution (1+9) of 500 µl whole blood with a 1-butanol (2%w/v), EDTA (0·05%w/v), Triton X-100 (0·05%w/v), NH4OH (1%w/v) solution containing Sc, Ge, Rh and Ir as internal standards. Using these methods, the laboratory has obtained successful results in external quality assessment schemes organized by the Institute for Occupational, Environmental and Social Medicine of the University of Erlangen, Germany (G-EQUAS program), and by the Institut National de Santé Publique, Québec (PCI and QMEQAS programs). For urine, a value of half the limit of detection (LOD), as determined previously,30 was attributed for concentrations below the LOD, but four elements (Be, In, Pt, Bi) for which nearly all values were below the LOD, were ignored. In blood, only Mn, Co, Cd, Hg and Pb are reported, because concentrations of Pd, Tl and U were nearly all below the LOD.
Metal concentrations in urine were expressed as µg/g creatinine to account for urine dilution. Creatinine was determined by a modified Jaffé reaction using a C502 module on a Cobas 8000 analyser (Roche diagnostics, Rotkreuz, Switzerland). Creatinine was not measured because of insufficient urine in one sample from an exposed child. Six participants (one control adult, one 3 y-old control child, four exposed children of 1 to 7 y) had concentrations of creatinine below 0.3 g/L, and four adult men (two diggers and two residents, including a man who had worked in a mine on the day of the survey) had concentrations of creatinine above 3 g/L. As recommended,55 the latter 10 urine samples were excluded, giving a total of 111 urine samples for statistical analysis. Blood was available for three of the subjects whose urinary data were excluded (one control adult; one exposed child, one exposed adult).
In 34 urine samples of the 36 residents recruited in the second campaign, we measured the concentration of 8-hydroxydeoxyguanosine (8OHdG), an index reflecting oxidative DNA damage, using an ELISA kit (JaICA, Nikken Seil Co, Shizuoka, Japan), as previously described.56 (We did not measure 8OHdG in the urine samples of the first campaign because these had not been stored adequately after the metal measurements).
The water and dust samples were analysed in the Division of Water and Soil Management of the KU Leuven. After aqua regia digestion of dust samples, as described previously,35 23 trace elements were quantified using an Agilent 7700x instrument. A value of half the limit of quantification (LOQ), as obtained in each run, was attributed when the concentration was below the LOQ, except for Se, Cd and Sn, which were ignored since nearly all their concentrations were below the LOQ in dust samples. For all measured elements (except for Zn and Zr), concentrations were similar between paired outdoor and indoor samples obtained from the same plot. Therefore, the average value of the metal concentrations measured in indoor and outdoor surface dust in each plot was considered representative for the exposure to surface dust for all the residents in that plot. The concentrations of metals in dust samples are expressed as µg/g dust [equivalent to parts per million (ppm)], rounded to three significant digits.
Data analyses
As the distributions of metal concentrations and 8OHdG concentrations were right-skewed, summary results are presented as geometric means with their 95% confidence intervals (CI). For the association analyses, we used the natural-log transformed concentrations of metals and of 8OHdG. Linear regression models were used to obtain crude GM ratios and their 95% CI; adjusted GM ratios and their 95% CI were obtained using mixed regression models adjusted for age and sex (fixed effects) and plot (random effect). Because plot K contained only two participants, we merged this plot with the nearby plot J for the latter analysis. In addition, we did stratified analyses by age: children (<14 years old) and adults (≥14 years old. Data analysis was conducted with STATA SE 14.0 statistical software (Stata Corporation, College Station, TX, USA). The level of statistical significance was set at p<0.05 (two-sided).
Supplementary Material
Acknowledgements
We thank Mrs Charlotte Cime Jinga, (former) mayor of Kolwezi, other local authorities, Mr Alphonse Makula and other local collaborators for their support and assistance during the surveys, Dr. Jacques Van Damme for his help and support. We thank Gladys Deumer, William Claassen and Kristin Coorevits for having measured trace elements by ICP-MS.
No specific funding was received for this study. The costs of metal analyses were covered by a VLIR-UOS grant (ZRDC2015PR090 to ES, CBLN and BN) and by IDEWE (External Service for Prevention and Protection at Work, Heverlee, Belgium). The costs of the 8OHdG measurements were covered by a European Research Council grant to TSN (ERC-2012-StG 310898). BN analysed the data and wrote the manuscript during a sabbatical leave (supported by grant K8.004.16N from FWO-Vlaanderen) at the Centre for Research in Environmental Epidemiology (CREAL), now Barcelona Institute for Global Health (ISGlobal).
Footnotes
Data availability
The (fully anonymized) data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
CBLN and BN conceived the study, did the fieldwork, collected the samples and wrote the manuscript. TKK, PMO and DKWM assisted with the processing of samples. LC performed the statistical analyses. VH was responsible for the analyses of metals in human samples. ES was responsible for the analyses of metals in environmental samples. NDS performed the measurements of 8OHdG under the supervision of TSN. TDP gave advice on geology and policy issues. JMLI gave advice on geology. OLN gave advice on child health. All authors gave input to successive drafts of the manuscript and approved its final version. CBLN and BN had full access to all the data.
Competing interests statement
None of the authors has conflicts of interest to declare in relation to this study.
References
- 1.Harper EM, Kavlak G, Graedel TE. Tracking the metal of the Goblins: Cobalt’s cycle of use. Environ Sci Technol. 2012;46:1079–1086. doi: 10.1021/es201874e. [DOI] [PubMed] [Google Scholar]
- 2.Cobalt Institute. Cobalt uses. [Accessed 22/02/2018];2017 Available at https://www.cobaltinstitute.org/
- 3.Shedd KB, Mccullough EA, Bleiwas DI. Global trends affecting the supply. Mining Engineering. 2017;69:37–42. [Google Scholar]
- 4.Anonymous. Digging for blue. Electric cars have made this once obscure metal the hottest commodity of 2017. [Accessed 22/02/2018];Quartz. 2017 Available at: https://qz.com/1159341/electric-cars-have-made-the-once-obscure-metal-cobalt-the-hottest-commodity-of-2017/ [Google Scholar]
- 5.Schmidt T, Buchert M, Schebek L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour Conserv Recycl. 2016;112:107–122. [Google Scholar]
- 6.Olivetti EA, Ceder G, Gaustad GG, Fu X. Lithium-ion battery supply chain considerations: Analysis of potential bottlenecks in critical metals. Joule. 2017;1:229–243. [Google Scholar]
- 7.European Commission. Critical Raw Materials - European Commission. [Accessed 22/02/2018];GROWTH. Internal Market, Industry, Entrepreneurship and SMEs. 2017 Available at: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en. [Google Scholar]
- 8.Federal Register. Final List of Critical Minerals 2018. [Accessed 16/07/2018]; Available at: https://www.federalregister.gov/documents/2018/05/18/2018-10667/final-list-of-critical-minerals-2018.
- 9.Wilburn DR. Cobalt mineral exploration and supply from 1995 through 2013. [Accessed 22/02/2018];US Geological Survey Scientific Investigations Report 2011-5084. 2012 Available at http://pubs.usgs.gov/sir/2011/5084/.
- 10.The World Bank. World Development Indicators. Congo, Dem. Rep; Washington, DC: 2016. [Accessed 22/02/2018]. Available at data.worldbank.org/country/congo-dem-rep#cp-wdi. [Google Scholar]
- 11.The World Bank. Worldwide Governance Indicators. Washington, DC: 2015. [Accessed 22/02/2018]. Available at http://info.worldbank.org/governance/wgi/index.aspx#reports. [Google Scholar]
- 12.Hsu A, et al. 2016 Environmental Performance Index. Yale University; New Haven: 2016. [Accessed 22/02/2018]. CT. 1-123. Available at http://epi2016.yale.edu/reports/2016-report. [Google Scholar]
- 13.Milesi JP, et al. An overview of the geology and major ore deposits of Central Africa: Explanatory note for the 1:4,000,000 map ‘Geology and major ore deposits of Central Africa’. J African Earth Sci. 2006;44:571–595. [Google Scholar]
- 14.Banza CLN, et al. High human exposure to cobalt and other metals in Katanga, a mining area of the Democratic Republic of Congo. Environ Res. 2009;109:745–752. doi: 10.1016/j.envres.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 15.De Putter T, Decrée S, Banza Lubaba Nkulu CNB. Mining the Katanga (DRC) Copperbelt: geological aspects and impacts on public health and the environment - towards a holistic approach. In: Kribek B, editor. IGCP/SIDA Project 594, Inaugural Workshop; Kitwe, Zambia. Czech Geological Survey; 2011. [Accessed 22/02/2018]. pp. 14–17. Available at www.geology.cz/igcp594/kitwe/PROCEEDINGS-OF-THE%20WORKSHOP.pdf. [Google Scholar]
- 16.Cuvelier J. Work and Masculinity in Katanga’s Artisanal Mines. Africa Spectrum. 2014;49:3–26. [Google Scholar]
- 17.Elenge MM, De Brouwer C. Identification of hazards in the workplaces of artisanal mining in Katanga. Int J Occup Med Environ Health. 2011;24:57–66. doi: 10.2478/s13382-011-0012-4. [DOI] [PubMed] [Google Scholar]
- 18.Amnesty International. "This is what we die for." Human rights abuses in the Democratic Republic of the Congo power the global trade in cobalt. London: Amnesty International; London, UK: 2016. [Accessed 22/02/2018]. Available at https://www.amnesty.org/es/documents/afr62/3183/2016/en/. [Google Scholar]
- 19.Tsurukawa N, Prakash S, Manhart A. Social impacts of artisanal cobalt mining in Katanga, Democratic Republic of Congo. Öko-Institut; Freiburg, Germany: 2011. [Accessed 22/02/2018]. Available at www.oeko.de/oekodoc/1294/2011-419-en.pdf. [Google Scholar]
- 20.BGR. [Accessed 16/07/2018];Cobalt from the DRC – Potential, Risks and Significance for the Global Cobalt Market (translated, original in German). Commodity Top News v. 53. 2017 Available at https://www.deutsche-rohstoffagentur.de/DE/Gemeinsames/Produkte/Downloads/Commodity_Top_News/Rohstoffwirtschaft/53_kobalt-aus-der-dr-kongo_en.pdf?__blob=publicationFile&v=6.
- 21.Frankel TC. The cobalt pipeline. Tracing the path from deadly hand-dug mines in Congo to consumers’ phones and laptops. [Accessed 22/02/2018];The Washington Post. 2016 Available at https://www.washingtonpost.com/graphics/business/batteries/congo-cobalt-mining-for-lithium-ion-battery/ [Google Scholar]
- 22.CME (Canadian Ministry of the Environment) Soil, Ground Water and Sediment Standards for Use under Part XV.1 of the Environmental Protection Act (PIBS#7382e01) [Accessed 22/02/2017];2011 Available at https://www.ontario.ca/page/soil-ground-water-and-sediment-standards-use-under-part-xv1-environmental-protection-act.
- 23.WHO (World Health Organization) Guidelines for drinking-water quality. Fourth edition. 2011. [Accessed 22/02/2018]. Available at www.who.int/water_sanitation_health/publications/2011/9789241548151_toc.pdf. [Google Scholar]
- 24.Gibb H, O’Leary KG. Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: A comprehensive review. Environmental Health Perspectives. 2014;122:667–672. doi: 10.1289/ehp.1307864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dooyema CA, et al. Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ Health Perspect. 2012;120:601–607. doi: 10.1289/ehp.1103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACGIH (American Conference of Governmental Industrial Hygienists) 2015 TLVs® and BEIs®. Threshold Limit Values for Chemical Substances and Physical Agents. Biological Exposure Indices. 2015. [Google Scholar]
- 27.Lison D Cobalt. In: Handbook on the Toxicology of Metals (4th Edition). Volume II: Specific Metals. Nordberg GF, Fowler BA, Nordberg M, editors. Academic Press - Elsevier; 2015. pp. 743–763. [Google Scholar]
- 28.Lison D, Buchet JP, Swennen B, Molders J, Lauwerys R. Biological monitoring of workers exposed to cobalt metal, salt, oxides, and hard metal dust. Occup Environ Med. 1994;51:447–450. doi: 10.1136/oem.51.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CDC (Centers for Disease Control and Prevention) Fourth National Report on Human Exposure to Environmental Chemicals, 2009. 2015. Updated tables, February 2015. [Google Scholar]
- 30.Hoet P, Jacquerye C, Deumer G, Lison D, Haufroid V. Reference values and upper reference limits for 26 trace elements in the urine of adults living in Belgium. Clin Chem Lab Med. 2013;51:839–849. doi: 10.1515/cclm-2012-0688. [DOI] [PubMed] [Google Scholar]
- 31.Nisse C, et al. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: The IMEPOGE study, 2008–2010. Int J Hyg Environ Health. 2017;220:341–363. doi: 10.1016/j.ijheh.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Mees F, et al. Concentrations and forms of heavy metals around two ore processing sites in Katanga, Democratic Republic of Congo. J African Earth Sci. 2013;77:22–30. [Google Scholar]
- 33.André G, Godin M. Child labour, agency and family dynamics: The case of mining in Katanga (DRC) Childhood. 2014;21:161–174. [Google Scholar]
- 34.Landrigan PJ, Kimmel CA, Correa A, Eskenazi B. Children’s health and the environment: public health issues and challenges for risk assessment. Environ Health Perspect. 2004;112:257–65. doi: 10.1289/ehp.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheyns K, et al. Pathways of human exposure to cobalt in Katanga, a mining area of the D.R. Congo. Sci Total Environ. 2014;490:313–321. doi: 10.1016/j.scitotenv.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 36.US EPA. EFH Highlights Chapter 5. Ingestion of soil and dust. [Accessed 16/07/2018]; Available at https://www.epa.gov/expobox/efh-highlights-chapter-5.
- 37.Orloff KG, et al. Human exposure to uranium in groundwater. Environ Res. 2004;94:319–326. doi: 10.1016/S0013-9351(03)00115-4. [DOI] [PubMed] [Google Scholar]
- 38.Hao Z, et al. Levels of rare earth elements, heavy metals and uranium in a population living in Baiyun Obo, Inner Mongolia, China: A pilot study. Chemosphere. 2015;128:161–170. doi: 10.1016/j.chemosphere.2015.01.057. [DOI] [PubMed] [Google Scholar]
- 39.Lourenço J, et al. Biomonitoring a human population inhabiting nearby a deactivated uranium mine. Toxicology. 2013;305:89–98. doi: 10.1016/j.tox.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Baker MG, et al. Blood manganese as an exposure biomarker: State of the evidence. J Occup Environ Hyg. 2014;11:210–217. doi: 10.1080/15459624.2013.852280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loft S, Fischer-Nielsen A, Jeding IB, Vistisen K, Poulsen HE. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 42.Barregard L, et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine. Antioxid Redox Signal. 2013;18:2377–2391. doi: 10.1089/ars.2012.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paustenbach DJ, Tvermoes BE, Unice KM, Finley BL, Kerger BD. A review of the health hazards posed by cobalt. Critical Reviews in Toxicology. 2013;43:316–362. doi: 10.3109/10408444.2013.779633. [DOI] [PubMed] [Google Scholar]
- 44.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. The Lancet Neurology. 2014;13:330–338. doi: 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinocourt C, Legrand M, Dublineau I, Lestaevel P. The neurotoxicology of uranium. Toxicology. 2015;337:58–71. doi: 10.1016/j.tox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Faber B, Krause B, Sánchez De La Sierra R. Artisanal Mining, Livelihoods, and Child Labor in the Cobalt Supply Chain of the Democratic Republic of Congo. Center for Effective Global Action; 2017. [Accessed 22/02/2018]. Policy Report. Available at cega.berkeley.edu/assets/cega_research_projects/179/CEGA_Report_v2.pdf. [Google Scholar]
- 47.Squadrone S, et al. Human exposure to metals due to consumption of fish from an artificial lake basin close to an active mining area in Katanga (D.R. Congo) Sci Total Environ. 2016;568:679–684. doi: 10.1016/j.scitotenv.2016.02.167. [DOI] [PubMed] [Google Scholar]
- 48.Bridge G. Contested terrain: mining and the environment. Annu Rev Environ Resour. 2004;29:205–259. [Google Scholar]
- 49.De Putter Thierry, Decrée S. Le potentiel minier de la République démocratique du Congo (RDC): mythes et composantes d’une dynamique minière. In: Marysse S, Omasombo J, editors. Conjonctures Congolaises 2012 : Politique, Secteur Minier et Gestion des Ressources Naturelles en RDC. MRAC, L’Harmattan; 2013. pp. 47–62. [Google Scholar]
- 50.Trefon T. Congo’s Environmental Paradox – Potential and Predation in a Land of Plenty. Zed Books; 2016. [Google Scholar]
- 51.Geenen S. A dangerous bet: The challenges of formalizing artisanal mining in the Democratic Republic of Congo. Resour Policy. 2012;37:322–330. [Google Scholar]
- 52.Hilson G, Hilson A, Maconachie R, McQuilken J, Goumandakoye H. Artisanal and small-scale mining (ASM) in sub-Saharan Africa: Re-conceptualizing formalization and ‘illegal’ activity. Geoforum. 2017;83:80–90. [Google Scholar]
- 53.De Putter T, Delvaux C. Certifier les ressources minérales critiques dans la région des Grands lacs. Politique Etrangère. 2013;78:99–112. [Google Scholar]
- 54.Trefon T, De Putter T. Ressources Naturelles et Développement: le Paradoxe Congolais. MRAC/L’Harmattan; 2017. [Google Scholar]
- 55.Cocker J, Mason HJ, Warren ND, Cotton RJ. Creatinine adjustment of biological monitoring results. Occup Med (Lond) 2011;61:349–353. doi: 10.1093/occmed/kqr084. [DOI] [PubMed] [Google Scholar]
- 56.Sughis M, Nawrot TS, Haufroid V, Nemery B. Adverse health effects of child labor: High exposure to chromium and oxidative DNA damage in children manufacturing surgical instruments. Environ Health Perspect. 2012;120:1469–1474. doi: 10.1289/ehp.1104678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.