Table 1.
Optimization of enantioselective Diels-Alder reactiona
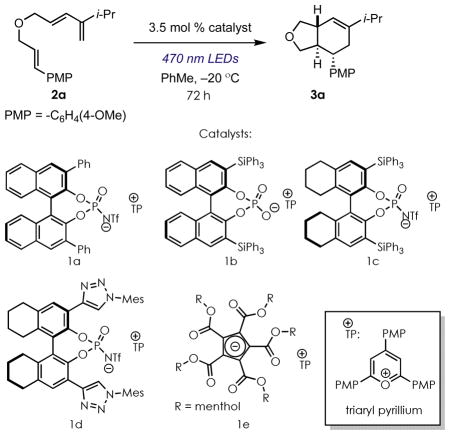
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Solvent | Yield[%]b | d.r.b | e.r.c |
| 1 | 1a | PhMe | <5 | -- | -- |
| 2 | 1b | PhMe | <5 | -- | -- |
| 3 | 1c | PhMe | 72 | 6:1 | 75:25 |
| 4 | 1c | DCM | 44 | 10:1 | 50:50 |
| 5 | 1c | THF | <5 | -- | -- |
| 6 | 1c | Et2O | <5 | -- | -- |
| 7 | 1d | PhMe | <5 | -- | -- |
| 8d | 1e | PhMe | <5 | -- | -- |
| 9d,e | 1e | DCM | 53 | 6:1 | 51:49 |
| 10d | 1e | PhCF3 | 25 | 6:1 | 50:50 |
| 11d | 1e | DCM:PhMe (9:1) | 25 | 6:1 | 53:47 |
Reactions were carried out in dry PhMe [0.2 M] in a cooling bath, with strips of blue LEDs (λmax = 470 nm) coiled around the reactions (see supporting information for details).
Determined by 1H NMR spectroscopic analysis of the crude reaction mixture relative to the internal standard (Me3Si)2O.
Determined by HPLC analysis of purified material using a chiral stationary phase.
Reaction performed using a 2.5 mol % catalyst loading.
Reaction performed at −30 °C.
