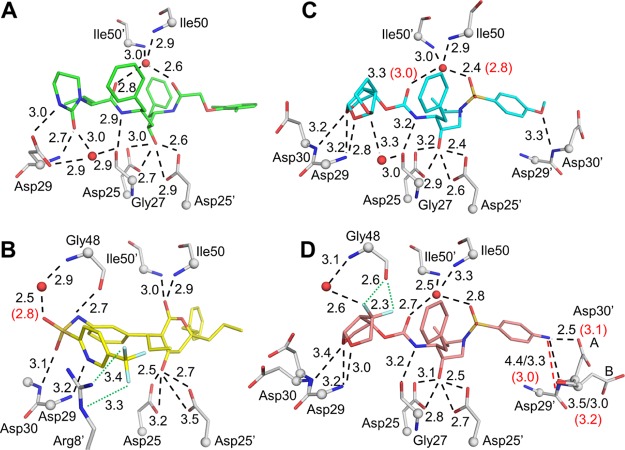
Figure 3.
Polar interactions of PRL76V with inhibitors. (A) Inhibitor 2 (green); (B) inhibitor 3 (yellow); (C) inhibitor 4 (cyan); and (D) inhibitor 5 (salmon). PR residues are shown in gray sticks with alpha-carbons as spheres; nitrogen (blue), oxygen (red), fluorine (pale cyan), water (red spheres). Side chains without polar interactions with inhibitors are omitted. Hydrogen bond interactions conserved in wild-type and mutant PR are shown as black dashed lines. Interactions that do not form in the mutant are shown as red dashed lines. Halogen bonds are in green dotted lines. Interatomic distances are given in Å for PRL76V (black) and PRWT (red in parenthesis) if values differ by 0.3 Å or more. In (D), Asp30 and Asp30′ are shown in two alternate conformations, and the interactions of Asp30′ with P2′ group of inhibitors differ for the alternate conformations. Distances for the (B) conformation of Asp30′ are shown after a forward slash.