ABSTRACT
Introduction: Functionalised carbon nanotubes (CNTs) have been shown to be promising biomaterials in neural systems, such as CNT -based nerve scaffolds to drive nerve regeneration. CNTs have been shown to modulate neuronal growth and improve electrical conductivity of neurons.
Methods: Cultured astrocytes on the functionalized CNTs (PEG, caroboxyl group) were assessed for distribution of GABA, glutamate uptake assay using isotope and change of conductance of CNTs by ATP. Immunostaining of GABA using anti-GABA (red), anti-GFAP (green) antibody in primary cortical astrocytes on MW-CNT and PDL coverslips.
Results: The functionalization of CNTs has improved their solubility and biocompatibility and alters their cellular interaction pathways. Recently, CNTs have been shown to modulate morphofunctional characteristics of glia as well as neurons. Among the various types of glia, astrocytes express diverse receptors for corresponding neurotransmitters and release gliotransmitters, including glutamate, adenosine triphosphate, and γ-amino butyric acid. Gliotransmitters are primarily released from astrocytes and play important roles in glia–neuron crosstalk.
Conclusion: This review focuses on the effects of CNTs on glial cells and discusses how functionalized CNTs can modulate morphology and gliotransmitters of glial cells. Based on exciting new findings, they look to be a promising material for use in brain disease therapy or neuroprosthetics.
KEYWORDS: Carbon nanotubes, astrocyte, gliotransmitter
1. Introduction
Since the first observation of carbon nanotubes (CNTs) by Iijima [1], unique structural, electrical, and mechanical properties of CNTs have been exploited in various fields and applications, such as biosensors [2], energy storage [3], drug delivery [4], and neural prosthetics [5]. CNTs are hollow cylinders made of graphene sheets rolled in on themselves to form a tube [6]. They are categorized based on the number of carbon layers assembled together: single-walled (SW-CNTs), double-walled (DW-CNTs), and multi-walled (MW-CNTs) [7]. The size of CNTs typically ranges from 0.4 to 2 nm in diameter for SW-CNTs and from 2 to 100 nm for MW-CNTs, while their length can vary from one to several hundred micrometers [8,9]. Both SW-CNTs and MW-CNTs possessing high tensile strengths are ultra-lightweight and have chemical stability and excellent thermal properties [10]. The biocompatibility and low cytotoxicity of CNTs are attributed to size, dose, duration, testing systems, and surface functionalization. The functionalization of CNTs improves their biocompatibility and solubility and alters their cellular interaction pathways, resulting in much reduced cytotoxic effects [11,12]. In particular, CNTs also have shown much promise in neural applications promoting neuronal function. CNT planatr strata have been shown to modulate neuronal growth and neurite outgrowth in culture and can affect the electrical properties of neurons, that is, direct physical interactions between the CNTs embedded within the film and the neurons grown on it [13]. In addition, CNTs have been shown to stimulate neurons efficiently and have long-term stability, while eliciting a significantly reduced inflammatory response in Parkinsonian rodents [14]. Recently, CNTs have been shown to modulate morpho-functional characteristics of glia as well as neurons [15,16]. Among the various types of glia, astrocytes occupy the greatest proportion (about 50%) in the brain and can make bidirectional communication with neurons. Also, astrocytes express diverse receptors for corresponding neurotransmitters and release gliotransmitters, including glutamate, adenosine triphosphate (ATP), and γ-amino butyric acid (GABA) [17,18]. Gliotransmitters are released from astrocytes and play important roles in modulating neuronal activity through neuron–glia interaction. Moreover, gliotransmitters have been shown to control synapse development, and their release can lead to paracrine actions on astrocytes [19]. For instance, released glutamate from astrocytes is Ca2+-dependent signaling and activates extrasynaptic neuronal N-methyl-D-aspartate (NMDA) receptors, resulting in an increase in the frequency of excitatory post-synaptic currents (EPSCs) and control of synaptic transmission [19,20]. In addition, vesicular release of astrocytic ATP can directly activate neuronal excitatory signaling through purinergic P2X receptors. It means released gliotransmitter from astrocytes is important to modulate or activate neuronal signaling through neuron–glia communication. Furthermore, neuron–glia communication through gliotransmitters is a crucial role of brain function. Released ATP from cortical astrocyte can effect induction of long-term potentiation (LTP) of synaptic plasticity in the neocortex [21]. In particular, gliotransmission is also related to function of neural activity and brain disease, which is an abnormality of neural networks. In normal conditions, tonic inhibition dominates more for regulating neuronal excitability than phasic inhibition, and astrocytic GABA mediates tonic inhibition through a direct permeable channel, bestrophin 1 (Best1) [18,22]. Reactive astrocytic GABA expressed high contents in the dentate gyrus, which resulted in impairment of LTP and memory by elevating tonic inhibition in an Alzheimer’s disease (AD) mouse model [23]. Jo et al. [24] also determined that abnormal release of astrocytic GABA impaired spike probability by monoamine oxidase-B (MAOB), but they observed that a reduction of MAOB rescued synapse plasticity and recovered learning and memory. Gliotransmitter is important to integral molecules of neural networks during disease and normal conditions. Therefore, this review focuses on the effects of CNTs on glial cells and gliotransmitters. It is very important to understand the glial biology on CNT for neural applications for brain disease therapy and prosthetics, such as brain–machine interfaces (BMI). Thus, we discuss how functionalized CNTs can modulate morphology and gliotransmitter of glial cells.
2. CNTs modulate morphology of glial cells
Pristine CNTs have a hydrophobic property and toxicity about cells and tissues, therefore a lot of studies have used various functionalized CNTs. In this review, we investigated how CNTs modulate the morphology and proliferation of glial cells (Table 1).
Table 1.
Functionalized CNTs modulate morphology of primary cultured mouse cortical astrocytes.
| SW-CNTs |
MW-CNTs |
|||
|---|---|---|---|---|
| 5 μg/ml | 60 nm | 50 nm | 1,000 nm | |
| CNT properties | Solution | Thickness | Length | Length |
| Functional group | PEG | PEG | -COOH | -COOH |
| Relative roundness | − | + | + | NS |
| Relative proliferation | NS | ++ | ++ | ++ |
| GFAP immunoreactivity | + | + | + | + |
Summary table for morphology of functionalized CNTs compared with astrocytes on control (PEI or PDL). SW-CNT-PEG (5 μg/ml) is treated on a PEI-coated coverslip, while 50 nm, 1,000 nm of MW-CNTs are a coated monolayer on a coverslip. NS (not significant) indicates no change. + indicates a significant increase (p < 0.05); ++ (p < 0.01), while − indicates a significant decrease (p < 0.05).
Based on astrocyte roundness factor, we assessed astrocyte morphology using morphometic parameters (roundness factor). The roundness factor formula is 4. The circularity of a circle is unity, while thin thread is approximately zero. SW-CNT-PEG (polyethylene glycol) has water solubility and biocompatibility materials. SW-CNT-PEG (5 μg/ml), in this case, is PEI-coated coverslips in the presence of SW-CNT-PEG colloidal solutes, and SW-CNT-PEG 60 nm is a coated 60 nm-thick film type of SW-CNT. Astrocytes on 60 nm-thick SW-CNT-PEG have rounder shape and increased cell area compared with astrocytes on PEI-coated coverslips [15], while astrocytes treated with SW-CNT-PEG (5 μg/ml) have significantly decreased relative roundness [25]. We also found that astrocytes on MW-CNT-50 have an increased shape factor compared with astrocytes on poly-D-lysine (PDL) coverslips. It is consistent with SW-CNT-PEG 60 nm. Astrocytes on 1,000 nm-length MW-CNT coverslips were shown to have a similar shape to those on PDL coverslips (Figure 2). Moreover, we evaluated the toxicity and proliferation of primary cortical astrocytes on MW-CNTs (50 nm, 1,000 nm) using CCK-8 solution in which viable cells convert water-soluble tetrazolium salt to formazan using dehydrogenase. The 1-day period gives an initial astrocyte adhesion on MW-CNT (50 nm, 1,000 nm) and PDL coverslips and the 4-day period allows for proliferation of astrocytes seeded on MW-CNT and PDL coverslips. After seeding for 1 day, the cell viability of astrocytes on MW-CNT-50 was increased and the relative density of live cells on MW-CNT-50 was fourfold that of cells on PDL. After seeding for 4 days, most of the higher cell viability was observed on the MW-CNT-50, while most of the proliferation ratio was measured on MW-CNT-1,000. Moreover, astrocytes on SW-CNT-PEG (colloidal solutes, 60 nm thickness film) were assessed using calcein dye, a vital fluorescent dye. Treatment of astrocytes with SW-CNT 60 nm significantly increased proliferation, while astrocytes in SW-CNT-PEG (5 μg/ml) solute had no significant proliferation compared with astrocytes on PEI-coated coverslips. Cultured primary cortical astrocytes on SW-CNTs (colloidal solutes, 60 nm thickness film) or MW-CNTs (50 nm, 1,000 nm) have preferential adhesion on functionalized CNTs. It is certain that functionalized CNTs have long-term stability and can be active for neuron–glia communication.
Figure 2.
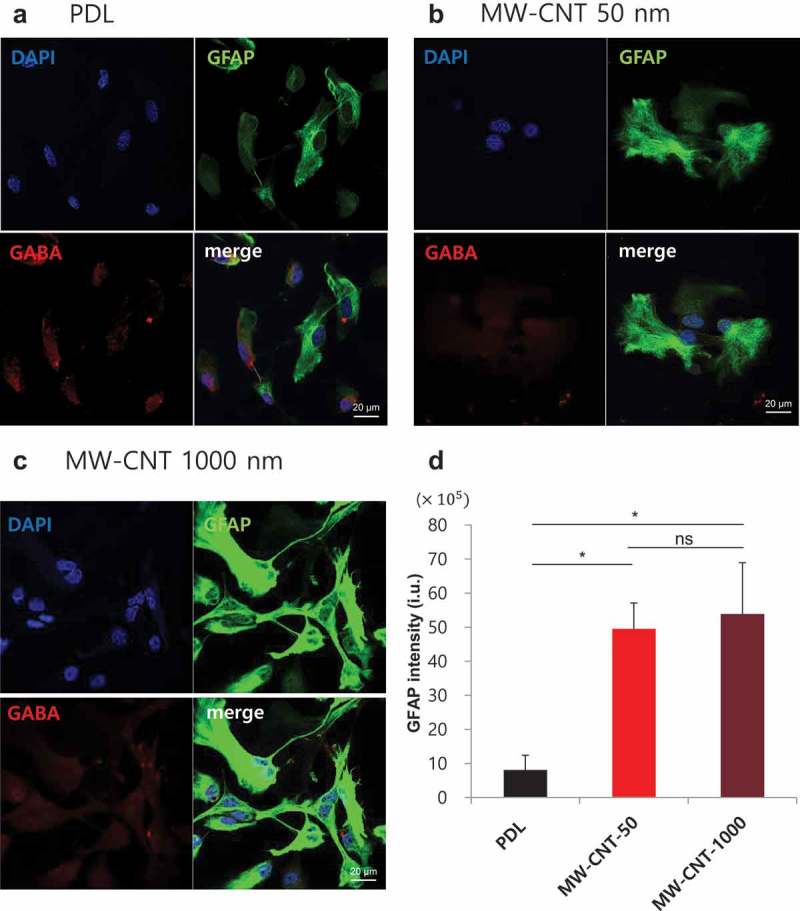
Glial GABA distribution on PDL and MW-CNTs (50 nm, 1,000 nm).
(a–c) Immunostaining of GABA using anti-GABA (red), anti-GFAP (green) antibody in primary cortical astrocytes on MW-CNT and PDL coverslips. Intracellular GABA in astrocytes on PDL is located near the nucleus, while astrocytes on MW-CNT 50 nm have a rounder shape compared with those on PDL. Astrocytes on MW-CNT 1,000 nm have more processes, which interact cell to cell. MW-CNT 50 nm and 1,000 nm affect distritubtuion of glial GABA. (d) Bar graph shows increased GFAP immunoreactivity of astrocytes on MW-CNTs (50 nm (n = 6), 1,000 nm (n = 6)) compared with PDL coverslips (n = 6), which have been cultured for 4 days. Scale bar: 20 μm.
In addition, we assessed the number of cell–cell interaction processes. Astrocytes can form gap junctions with neighboring cells, thereby forming interconnected groups of cells sharing a common cytoplasm [26]. We suggest that astrocytes on MW-CNTs (50 nm, 1,000 nm) have more cell–cell interaction (p < 0.05), with higher numbers of cell processes compared with those seen on PDL coverslips [16]. Some reports show that the cause of neurodegenerative diseases, such as Parkinson’s disease and AD, is a progressive loss of structure or function of neurons, as well as neuronal cell death. Also, increased glial fibrillary acidic protein (GFAP) immunoreactivity decreases tissue damage and neuronal loss and demyelination [27,28]. Astrocytes on functionalized CNTs significantly increased immunoreactivity of GFAP, an astrocyte-specific marker, using immunocytochemistry. Therefore, CNTs immobilized various functional groups or biocompatible materials having variable characteristics to glial cells. These results suggest that each of the characteristics of functionalized CNTs can appropriately be used in various brain disease therapies.
3. CNTs enhance glutamate uptake in astrocytes
Glutamate plays the principal role in neural activation and is the major excitatory neurotransmitter. It was proposed that glutamate acts postsynaptically on three families of ionotropic receptors, named after their preferred agonists, NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainite [29]. Glutamate is released from vesicles in presynaptic terminals by a Ca2+-dependent mechanism that involves voltage-dependent Ca2+ channels [30]. The glutamate–glutamine cycle is the principal metabolic pathway with an adequate supply of the neurotransmitter glutamate for maintenance in the central nervous system (CNS) [31]. When glutamate is taken up into glial cells by glutamate transporter, it is converted to glutamine and transported into the presynaptic neuron which is converted back into glutamate, and then transferred into vesicular glutamate transporter (VGLUT) [32]. Astrocytes release glutamine to be taken up into neurons for use as a precursor to the synthesis of glutamate or GABA [33]. Moreover, astrocytes take up extracellular glutamate primarily through the excitatory amino acid transporters GLAST/EAAT1 and GLT1/EAAT2, present on their surface [34]. Released glutamate causes an action potential and then glutamate in the extracellular space is cleared by glutamate transporters to maintain levels of glutamate, thereby terminating the synaptic transmission [32]. Without the activity of glutamate transporters, excessive amounts of glutamate acts as a toxin to neurons by triggering a number of biochemical cascades [35]. When the glutamate concentration around the synaptic cleft cannot be decreased or reaches higher levels, the neuron kills itself by a process called apoptosis, therefore excessive glutamate causes a pathologic phenomenon (e.g. ischemic stroke and spinal cord injury) [36,37]. Thus, expeditious removal of glutamate from the extracellular space is important for the survival and normal function of neurons. When functionalized CNTs implant in the brain, changing amounts of glutamate is critically important. Chemically functionalized water-soluble SW-CNT-PEG 5 μg/ml increases GFAP immunoreactivity [25] and GLAST, which is highly expressed in astrocytes [38]. Gottipati et al. [34] showed that SW-CNT-PEG upregulates the uptake of glutamate by astrocytes. Astrocyte plated into well-plates with SW-CNT-PEG 5 μg/ml for 4 days enhanced the uptake of glutamate (Figure 1). To identify whether glutamate is taken up into astrocytes by GLAST, they treated 100 μM TBOA (DL-threo-β-benzyloxyaspartic acid), which is a competitive EAAT blocker [39]. TBOA significantly blocked about 40% of the uptake of glutamate, and they additionally confirmed SW-CNT-PEG increased GLAST expression on the plasma membrane of astrocytes using immunocytochemistry. Astrocytes with SW-CNT-PEG 5 μg/ml have more efficient glutamate uptake, which can rapidly convert to glutamine from the extracellular space as well as modulate morphology. Therefore, the reduction of extracellular glutamate leads to decreased excitotoxicity, and functionalized CNTs can be used in brain therapy for excitotoxicity, such as epilepsy and stroke.
4. CNTs modulate the intracellular distribution of GABA in astrocytes
Since the 1950s, it has been known that GABA is the major inhibitory neurotransmitter in the CNS of vertebrates [40]. GABA acts at inhibitory synapses in the brain by binding to specific receptors in pre- and post-synaptic neurons. Recent studies have demonstrated a robust release of GABA from glial cells in human astrocytes and acute brain slices [18,41]. Furthermore, astrocytic release of GABA can cause tonic inhibition in several brain regions, including the thalamus and cerebellum [42,43]. Tonic inhibition orginates from the sustained activation of high affinity GABA receptors by ambient GABA [22]. In the cerebellum, some studies have reported that a Ca2+-activated anion channel, Best1, mediates tonic inhibition by releasing GABA through direct permeation [18,43]. In addition, glial MAOB is a key synthesizing enzyme of GABA and converts putrescine to GABA in the mitochondria of astrocytes [44]. In cultured O2A glial protenitor cells, they were able to synthesize GABA from putrescine. Barres et al. [45] showed a strong GABA immunoreacitivity, while there was no detectable GAD expression in O2A glial progenitor cells. Glial GABA transporter, which exists transmembrane, takes up GABA into astrocytes and can release the GABA by reversal of the GAT-1 expression [46]. In particular, GAT-2 and GAT-3 are expressed in astrocytes and mediate release of glial GABA by a reversal action [47]. Therefore, tonic inhibition releasing glial GABA is a critical role of physiological and pathological conditions. Based on these studies, we discuss functionalized CNTs which can implant in the brain for brain disease therapy and modulate GABA. We investigated intracellular GABA distribution on MW-CNT and PDL coverslips (Figure 1) [16]. PDL is a positively charged material which is generally used to attach cells on to culture dishes and used as a control group. In MW-CNTs, astrocytic GABA spreads into more cell processes than occurs on PDL coverslips (Figure 2). When astrocytic GABA spreads into cell processes from the cell body, GABA can be released more easily and in greater quantities compared with that from astrocytes on PDL coverslips. The diffusion of GABA into cell processes accelerates release to tripartite synapses and increases astrocyte–neuron interactions, implying increased bidirectional communication because proteins such as transporters, receptors and channels are involved in neurotransmitter release. Therefore, our future studies are required to demonstrate astrocyte–neuron interaction with MW-CNTs, which may generate therapy material for improving the quality of human life.
Figure 1.
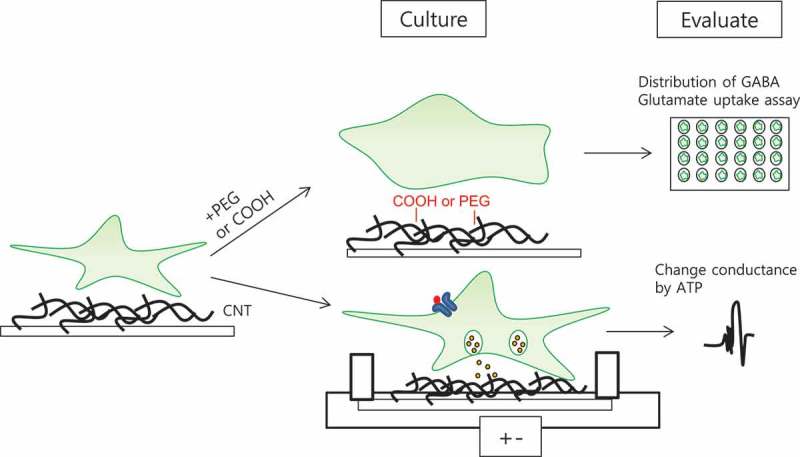
Scheme of experimental process of gliotransmitter on CNTs.
Cultured astrocytes on the functionalized CNTs (PEG, caroboxyl group) were assessed for distribution of GABA, glutamate uptake assay using isotope and change of conductance of CNTs by ATP.
5. CNTs can produce an active interaction region between triggered ATP release of astrocytes and neurons
ATP is a critical signaling molecule regulating many biological functions, such as neurotransmitters or neuromodulators in both the CNS and peripheral nervous system, as well as a universal energy carrier [48,49]. The released ATPs bind with ATP receptors on the adjacent neurons and astrocytes, leading to dissemination of a Ca2+ wave through the astrocyte–neuron network in the region [50]. ATP, an endogenous ligand of purinergic receptors, may directly mediate synaptic transmission as a fast neurotransmitter [51] or it may modulate synaptic efficacy as a neuromodulator. Zhang et al. [52] have shown that ATP released from astrocytes as a result of neuronal activity can also modulate central synaptic transmission. In cultures of hippocampal neurons, endogenously released ATP tonically suppresses glutamatergic synapses via presynaptic P2Y receptors [52]. In particular, P2Y receptor is a metatropic receptor first activated by ADP and neuroprotects in ischemic conditions [53]. Therefore, ATP is important to modulate neurotransmitters, but ATP signaling is not yet understood. Currently, the detection method of ATP release is luciferase assay, but it cannot distinguish whether ATP is released by non-specific cytolysis or a particular release mechanism. However, the SW-CNT network can be used to interface directly about living astrocytes and detect the triggered local ATP release from these cells (Figure 1). ATP released from astrocytes diffused on the SW-CNT network, which means the released ATP and SW-CNT network interact by interaction (Figure 3) [54]. Purinergic signaling is included in nervous regeneration following epilepsy-associated seizures in the brain [55], ischemia and neurodegenerative disorders [56]. Moreover, ATP can stimulate astrocyte proliferation, which contributes to hyperplastic responses, and P2Y receptor antagonists have been proposed as potential neuroprotective agents in the cortex. ATP, which presents in high concentrations within the brain (e.g. cortex, hippocampus) [57], co-releases with glutamate. Fujii et al. [58] showed cooperativity between extracellular ATP and NMDA receptors in long-term potentiation induction in hippocampal CA1 neurons. Therefore, we expect CNTs can produce a place to activate interaction with various gliotransmitters, such as glutamate, and GABA for brain disease therapy.
Figure 3.
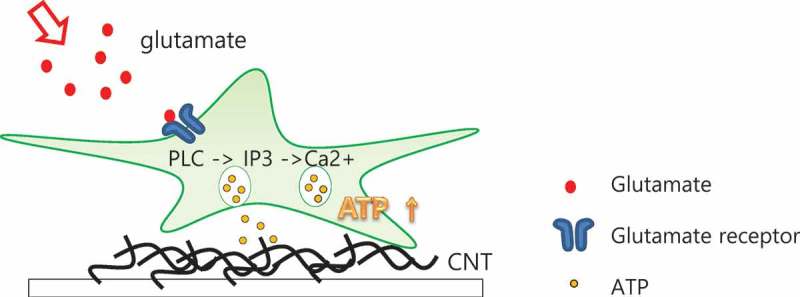
Illustration of active interaction region of CNTs through released ATP binding to CNT.
Released ATP from astrocytes diffused on the SW-CNT network, which means released ATP and the SW-CNT network interact by interaction.
6. Concluding remarks
This review highlights that functionalized SW-CNTs (colloidal solutes, 60 nm thickness film) or MW-CNTs can be applied to various neurodegenerative disease therapies. Unlike pristine CNTs, astrocytes on functionalized SW-CNTs or MW-CNTs have more adhesive characteristics and modulate morphology, interaction processes and GFAP immunoreactivity. Immobilized functional groups or materials each have characteristics that affect glial cells or gliotransmitters. Many studies have shown CNTs to be promising for nerve regeneration and can be applied as BMI-coated CNTs. Metal electrodes used BMI are inadequate prospects, such as poor electrochemical properties and high stiffness, which mean they have the low charge density and reduce stability. They can cause the risk of tissue damages by their electrical limitation. However, CNT fibers are suitable for recording single-neuron activity and have long-term stability [14]. In addition, various gliotransmitters in astrocytes treated with SW-CNTs or MW-CNTs have increasing uptake or release. Some studies have shown that ATP-induced stimulation of P2X7 receptors releases not only ATP and glutamate, but also GABA from astrocytes of the brain or Muller cells of the retina [59]. Gliotransmitters in astrocyte-affected CNTs have an active effect on neurodegenerative disease and neuron–glia crosstalk by transporter, signaling factor and receptor. However, CNTs have a controversial problem with toxicity and accumulation in bodies. Therefore, if functionalized CNTs are determined not to be posionous and to have biodegradable properties, future studies will be required to demonstrate the mechanism of glia and CNT interaction. Then CNT-based biomaterials need in vivo studies for bioapplications such as neural prostheses.
Responsible Editor Dr Aaron Tan, University College London, UK
Funding Statement
This work was supported by the Korea Foundation for the Advancement of Science and Creativity (KOFAC), and funded by the Korean Government (MOE).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Iijima S.Helical microtubules of graphitic carbon. Nature 1991; 354, 1–7. doi: 10.1038/354056a0 [DOI] [Google Scholar]
- [2].Chen RJ, Bangsaruntip S, Drouvalakis KA, et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc Natl Acad Sci U S A. 2003;100(9):4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Patchkovskii S, Tse JS, Yurchenko SN, et al. Graphene nanostructures as tunable storage media for molecular hydrogen. Proc Natl Acad Sci U S A. 2005;102(30):10439–10444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kam NW, O’Connell M, Wisdom JA, et al. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci U S A. 2005;102(33):11600–11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wei W, Sethuraman A, Jin C, et al. Biological properties of carbon nanotubes. J Nanosci Nanotechnol. 2007;7(4–5):1284–1297. [DOI] [PubMed] [Google Scholar]
- [6].Belyanskaya L, Weigel S, Hirsch C, et al. Effects of carbon nanotubes on primary neurons and glial cells. Neurotoxicology. 2009;30(4):702–711. [DOI] [PubMed] [Google Scholar]
- [7].Salvador-Morales C, Flahaut E, Sim E, et al. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43(3):193–201. [DOI] [PubMed] [Google Scholar]
- [8].Bekyarova E, Ni Y, Malarkey EB, et al. Applications of carbon nanotubes in biotechnology and biomedicine. J Biomed Nanotechnol. 2005;1(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Malarkey EB, Parpura V.. Applications of carbon nanotubes in neurobiology. Neurodegener Dis. 2007;4(4):292–299. [DOI] [PubMed] [Google Scholar]
- [10].Smart SK, Cassady AI, Lu GQ, et al. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- [11].Bhirde AA, Patel S, Sousa AA, et al. Distribution and clearance of PEG-single-walled carbon nanotube cancer drug delivery vehicles in mice. Nanomedicine (Lond). 2010;5(10):1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cui H-F, Vashist SK, Al-Rubeaan K, et al. Interfacing carbon nanotubes with living mammalian cells and cytotoxicity issues. Chem Res Toxicol. 2010;23(7):1131–1147. [DOI] [PubMed] [Google Scholar]
- [13].Cellot G, Cilia E, Cipollone S, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4(2):126–133. [DOI] [PubMed] [Google Scholar]
- [14].Vitale F, Summerson SR, Aazhang B, et al. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano. 2015;9(4):4465–4474. [DOI] [PubMed] [Google Scholar]
- [15].Gottipati MK, Samuelson JJ, Kalinina I, et al. Chemically functionalized single-walled carbon nanotube films modulate the morpho-functional and proliferative characteristics of astrocytes. Nano Lett. 2013;13(9):4387–4392. [DOI] [PubMed] [Google Scholar]
- [16].Min J-O, Kim SY, Shin US, et al. Multi-walled carbon nanotubes change morpho-functional and GABA characteristics of mouse cortical astrocytes. J Nanobiotechnol. 2015;13:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Volterra A, Meldolesi J.. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6(8):626–640. [DOI] [PubMed] [Google Scholar]
- [18].Lee S, Yoon B-E, Berglund K, et al. Channel-mediated tonic GABA release from glia. Science. 2010;330(6005):790–796. [DOI] [PubMed] [Google Scholar]
- [19].Halassa MM, Fellin T, Haydon PG.. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. [DOI] [PubMed] [Google Scholar]
- [20].Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 2010;72:335–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lalo U, Rasooli-Nejad S, Pankratov Y. Exocytosis of gliotransmitters from cortical astrocytes: implications for synaptic plasticity and aging. Biochem Soc Trans. 2014;42(5):1275–1281. [DOI] [PubMed] [Google Scholar]
- [22].Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–229. [DOI] [PubMed] [Google Scholar]
- [23].Wu Z, Guo Z, Gearing M, et al. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s disease model. Nat Commun. 2014;5:4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jo S, Yarishkin O, Hwang YJ, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20(8):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gottipati MK, Kalinina I, Bekyarova E, et al. Chemically functionalized water-soluble single-walled carbon nanotubes modulate morpho-functional characteristics of astrocytes. Nano Lett. 2012;12(9):4742–4747. [DOI] [PubMed] [Google Scholar]
- [26].Lee SH, Kim WT, Cornell-Bell AH, et al. Astrocytes exhibit regional specificity in gap-junction coupling. Glia. 1994;11(4):315–325. [DOI] [PubMed] [Google Scholar]
- [27].Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. [DOI] [PubMed] [Google Scholar]
- [28].Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–407. [DOI] [PubMed] [Google Scholar]
- [29].Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr. 2000;130:1007S–1015S. [DOI] [PubMed] [Google Scholar]
- [30].Birnbaumer L, Campbell KP, Catterall WA, et al. The naming of voltage-gated calcium channels. Neuron. 1994;13(3):505–506. [DOI] [PubMed] [Google Scholar]
- [31].Shen J. Modeling the glutamate-glutamine neurotransmitter cycle. Front Neuroenerget. 2013;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004;45(3):250–265. [DOI] [PubMed] [Google Scholar]
- [33].Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem. 2006;98:641–653. [DOI] [PubMed] [Google Scholar]
- [34].Gottipati MK, Bekyarova E, Haddon RC, et al. Chemically functionalized single-walled carbon nanotubes enhance the glutamate uptake characteristics of mouse cortical astrocytes. Amino Acids. 2015;47(7):1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 2005;1034(1–2):11–24. [DOI] [PubMed] [Google Scholar]
- [36].Ankarcrona M, Dypbukt JM, Bonfoco E, et al. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15(4):961–973. [DOI] [PubMed] [Google Scholar]
- [37].Hulsebosch CE, Hains BC, Crown ED, et al. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60(1):202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Storck T, Schulte S, Hofmann K, et al. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89(22):10955–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shimamoto K, Lebrun B, Yasuda-Kamatani Y, et al. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53(2):195–201. [DOI] [PubMed] [Google Scholar]
- [40].Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3(9):715–727. [DOI] [PubMed] [Google Scholar]
- [41].Lee M, McGeer EG, McGeer PL. Mechanisms of GABA release from human astrocytes. Glia. 2011;59(11):1600–1611. [DOI] [PubMed] [Google Scholar]
- [42].Jiménez-González C, Pirttimaki T, Cope DW, et al. Non-neuronal, slow GABA signalling in the ventrobasal thalamus targets δ-subunit-containing GABA(A) receptors. Eur J Neurosci. 2011;33(8):1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yoon B-E, Jo S, Woo J, et al. The amount of astrocytic GABA positively correlates with the degree of tonic inhibition in hippocampal CA1 and cerebellum. Mol Brain. 2011;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yoon B-E, Woo J, Chun Y-E, et al. Glial GABA, synthesized by monoamine oxidase B, mediates tonic inhibition. J Physiol. 2014;592(22):4951–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barres BA, Koroshetz WJ, Chun LL, et al. Ion channel expression by white matter glia: the type-1 astrocyte. Neuron. 1990;5(4):527–544. [DOI] [PubMed] [Google Scholar]
- [46].Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88(3):1407–1419. [DOI] [PubMed] [Google Scholar]
- [47].Héja L, Nyitrai G, Kékesi O, et al. Astrocytes convert network excitation to tonic inhibition of neurons. BMC Biol. 2012;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fields RD, Stevens B. ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000;23(12):625–633. [DOI] [PubMed] [Google Scholar]
- [49].Gourine AV, Llaudet E, Dale N, et al. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436(7047):108–111. [DOI] [PubMed] [Google Scholar]
- [50].Guthrie PB, Knappenberger J, Segal M, et al. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19(2):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359(6391):144–147. [DOI] [PubMed] [Google Scholar]
- [52].Zhang J-M, Wang H-K, Ye C-Q, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40(5):971–982. [DOI] [PubMed] [Google Scholar]
- [53].Sun -J-J, Liu Y, Ye Z-R. Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci Bull. 2008;24(4):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Johnson RR, Johnson AT, Klein ML. Probing the structure of DNA-carbon nanotube hybrids with molecular dynamics. Nano Lett. 2008;8(1):69–75. [DOI] [PubMed] [Google Scholar]
- [55].Wieraszko A, Goldsmith G, Seyfried TN. Stimulation-dependent release of adenosine triphosphate from hippocampal slices. Brain Res. 1989;485(2):244–250. [DOI] [PubMed] [Google Scholar]
- [56].Burnstock G. Introduction: ATP and its metabolites as potent extracellular agents. Curr Top Membr. 2003;54:1–27. [Google Scholar]
- [57].Kogure K, Alonso OF. A pictorial representation of endogenous brain ATP by a bioluminescent method. Brain Res. 1978;154(2):273–284. [DOI] [PubMed] [Google Scholar]
- [58].Fujii S, Sasaki H, Mikoshiba K, et al. A chemical LTP induced by co-activation of metabotropic and N-methyl-D-aspartate glutamate receptors in hippocampal CA1 neurons. Brain Res. 2004;999(1):20–28. [DOI] [PubMed] [Google Scholar]
- [59].Pannicke T, Fischer W, Biedermann B, et al. P2X7 receptors in Müller glial cells from the human retina. J Neurosci. 2000;20(16):5965–5972. [DOI] [PMC free article] [PubMed] [Google Scholar]


