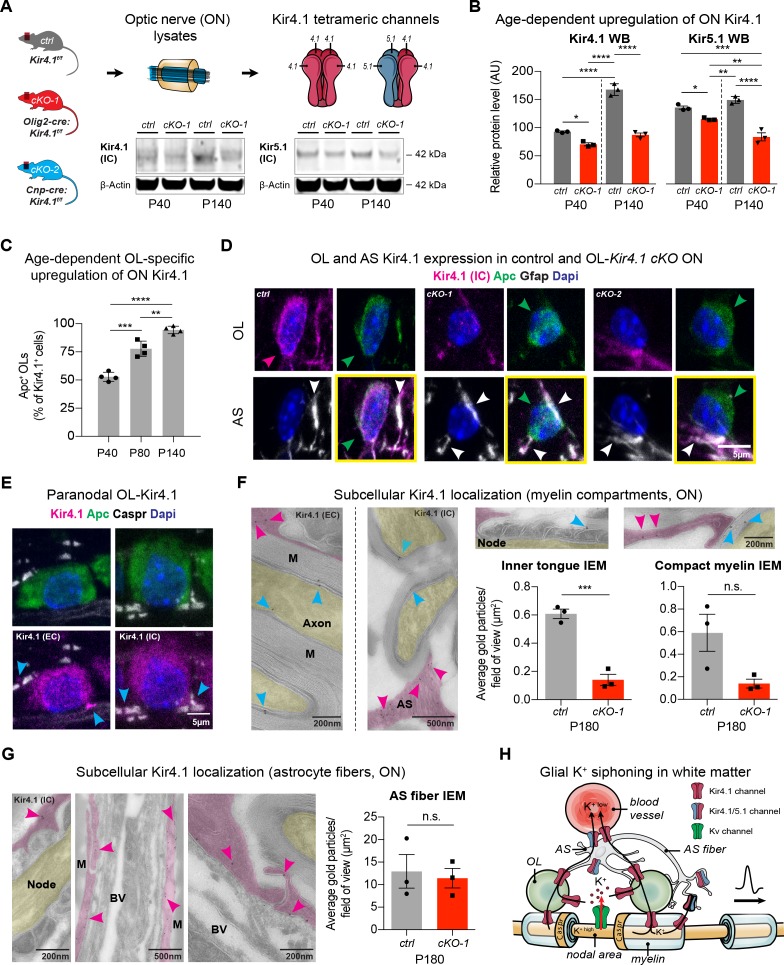
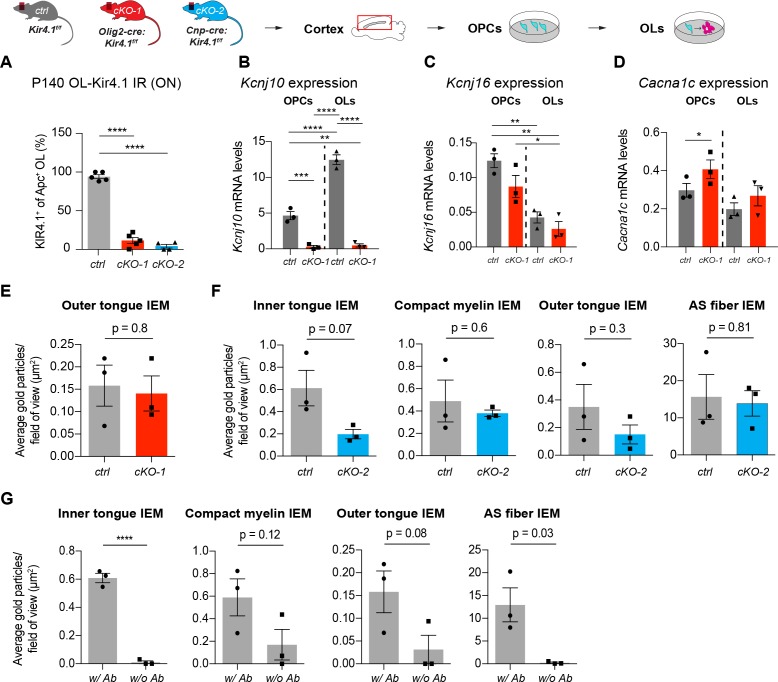
Figure 1. OL-Kir4.1 is upregulated during postnatal development and localized to peri-axonal spaces.
Kir4.1 ON protein levels were upregulated between age P40 and P140, whereas Kir5.1 protein levels did not change during aging (A–B). Note substantial loss of Kir4.1 protein in Olig2-cre driven Kcnj10 cKO (cKO-1) mice at P40, which became more apparent at P140; Kir5.1 protein was also reduced in cKO-1 ONs at P40 and P140 (control and cKO-1: n = 3 for all time points) (A–B). Quantification of Kir4.1+ Apc+ OLs confirmed age-dependent upregulation of OL-Kir4.1 channels between P40 and P140 (n = 4 for all time points) (C). One-way ANOVA with Tukey’s multiple comparison tests were performed in B and C; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. Kir4.1 channels were lost from both ON OL cell bodies in cKO-1 and Cnp-cre driven Kcnj10 cKO (cKO-2) mice versus controls (D). Note that Kir4.1+ OL are marked by magenta-colored arrowhead; Apc+ OLs are indicated by green arrowheads. Note AS Kir4.1 immunoreactivity and contacts of Kir4.1+ AS fibers with OLs (white arrowheads). Merged images are shown in panels highlighted by yellow surroundings (D). Kir4.1 was strongly expressed in OLs along spinal fiber tracts; note that cyan-colored arrowheads mark juxta-axonal Kir4.1 IR (E). Kir4.1 immunogold electron microscopy (IEM) labeling revealed presence of gold particles at inner and outer myelin tongue (cyan-colored arrowheads) and within AS fibers (magenda-colored arrowheads) adjacent to myelin sheaths (M = myelin) and blood vessels (BV = blood vessel; ctrl: n = 3, cKO-1: n = 3; F–G). Axon structures are highlighted in yellow, AS fibers are highlighted in magenta. Note decrease in inner tongue (F) but not compact myelin (F) or AS fiber (G) IEM labeling in cKO-1 ON tissue versus controls. Cartoon highlights proposed mechanism of glial K+ siphoning from axons during saltatory conduction towards blood vessels via a network of axonal Kv and glial Kir4.1 channels (H). Mann-Whitney tests were performed in F–G; ***p≤0.001, p=0.06 (F, compact myelin IEM), p=0.74 (G, AS fiber IEM). Data are presented as mean ±s.e.m in B–C and F–G.