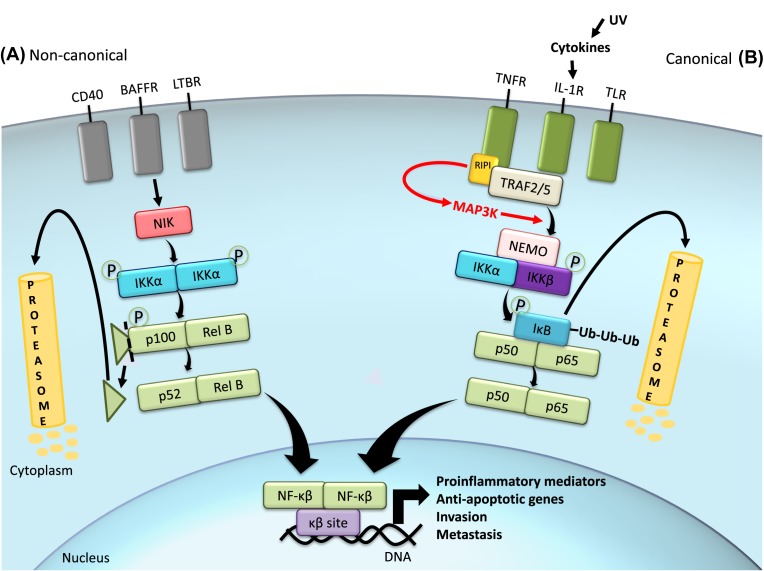
Figure 5. Canonical and non-canonical NF-κB signaling pathways.
The NF-κB cascade can be initiated by several types of receptors, which will trigger either the canonical or the non-canonical pathway. When the canonical pathway is initiated by receptors such as TNFR, the activated receptor interacts with associated proteins (TRAFs), which recruit RIP1 kinase to be phosphorylated by the receptor. This pathway culminates in the phosphorylation and activation of the IKK complex formed by scaffold protein NEMO, IKKα, and IKKβ. NF-κB dimers are conjugated with inhibitor IKB in the cytoplasm. IKK complex is responsible for phosphorylating IKB and therefore targetting it for degradation, releasing NF-κB to translocate into the nucleus and promote transcription of target genes by binding to the κB site in the DNA. The non-canonical pathway is initiated by other receptors, which relies on the activation of IKKα homodimer by the NF-κB inducing kinase (NIK), assisted by TRAF. p100:RelB, the most common NF-κB dimer in this pathway, is phosphorylated on the p100 subunit, which will be cleaved in its inhibitory domain and release active p52:RelB dimer to exert its nuclear functions. In melanoma, UV light exposure is one of the factors that might activate NF-κB by inducing cytokines that signals through this pathway to induce a pro-inflammatory response. Sustained activation of NF-κB also promotes the expression of anti-apoptotic genes, as well as invasion- and metastasis-related genes.