Figure 3.
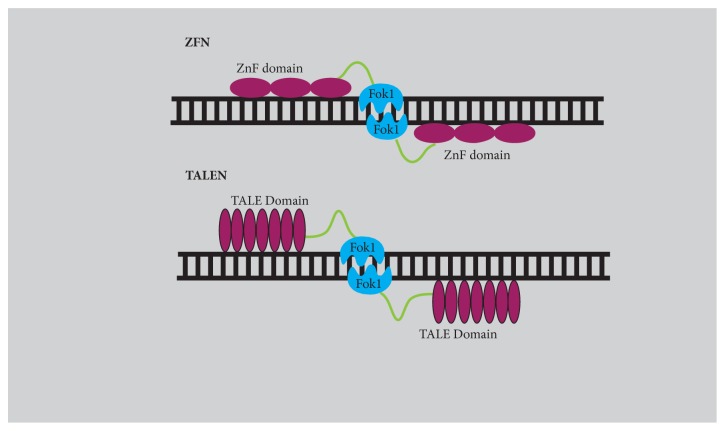
Zinc Finger Nuclease (ZFN). ZFN (discovered by Chandrasegaran and his team in 1996) are sequence-specific chimeric proteins containing DNA binding domain fused to nonspecific cleavage domain (derived from type II restriction enzyme FOKI) [18–20]. DNA binding domain consists of 3-6 Cys2-His2 tandemly arranged zinc finger repeats that recognize 9-18 bp sequences (3 bp by each ZFN unit) [21]. Each finger contains approximately 30 amino acids with one α helix and two β strands [19]. The chimeric ZFN are engineered to assemble in pairs and enable efficient and precise genetic modifications by inducing DSBs. In addition to dimerization of FokI nuclease domains, ZFN requires correct spacer sequence (5-6 bp) and orientation of chimeric nucleases for the cleavage of dsDNA [22, 23]. Despite wide applications, the major challenge was to increase the specificity since it was cleaving off-targets that had sequence homology to on-target making ZNF cytotoxic [24]. Custom designed ZNF are prepared by altering DNA binding domain and catalytic domain through mutagenesis and modular assembly of precharacterized ZNF [25]. Till date it has been used for genome editing in mice [26], insects [27], zebrafish [28], and humans (embryonic cells and induced pluripotent stem cells) [29]. Transcription activator-like effector Nuclease (TALEN). TALENS derived from the plant pathogen Xanthomonas sp. are virulence proteins which consist of DNA binding domain and FOK1 nuclease domain. These domains act as dimers and bind to the opposite strands of DNA, separated by a spacer sequence, and create a double-stranded break. DNA binding domain consists of 33-35 amino acid repeats and is arranged in tandem [30, 31]. These repeats are similar except for two highly variable amino acids at positions 12 and 13 called repeat variable di-residue (RVD) which are responsible for specific base recognition and engineering of these bases in repeats [32]. A total four of RVD modules can recognize each of the bases guanine (G), adenine (A), cytosine (C), and thymine (T) and each module is able to function independently. The target sequence must contain thymine (T) at the 5′ end for recognition by TALENS and the spacer sequence should be of 12-20 bp between the dimers [31]. Compared to ZNF, TALENS possess reduced cytotoxicity, are high on targeting efficiency, and are easy to design. However, its high molecular weight makes it difficult for the delivery in the nucleus. AAV vectors are generally used for the delivery of TALENs due to their low immunogenicity and less oncogenic risk [33]. TALENS have been applied for gene disruption in Drosophila [34], C. elegans [35], Arabidopsis [36], Zebrafish [37], and human embryonic stem cells [38].