Abstract
Background
Mongolia is one of the endemic countries for cystic echinococcosis (CE), a zoonotic disease caused by the larval stage of Echinococcus granulosus. The goal of this study is to describe the current clinical management of CE in Mongolia, to capture the distribution of cyst stages of patients treated, and to contrast current practice with WHO-IWGE expert consensus.
Methods
Hospital records of CE patients treated between 2008 and 2015 at the three state hospitals and fulfilling the inclusion criterion ‘discharge diagnosis CE’ (ICD 10 code B.67.0–67.9) were reviewed. Demographical, geographical, clinical and ultrasonography (US) data were extracted and analyzed. The annual surgical incidence was estimated. The digital copies of US cyst images were independently staged by three international experts following the WHO CE cyst classification to determine the proportions of patients which ideally would have been assigned to the WHO recommended treatment modalities surgery, percutaneous, medical (benzimidazole) treatment and watch & wait.
Results
A total of 290 patient records fulfilled the inclusion criteria of the study. 45.7% of patients were below 15 years of age. 73.7% of CE cysts were located in abdominal organs, predominantly liver. US images of 84 patients were staged and assessed for interrater-agreement. The average raw agreement was 77.2%. Unweighted Kappa coefficient and weighted Kappa was 0.57 and 0.59, respectively. Mean proportions of images judged as stages CE1, CE2, CE3a, CE3b, CE4 and CL were 0.59, 0.01, 0.19, 0.08, 0.03 and 0.11, respectively. 40 cysts met the inclusion criteria of treatment modality analysis. The mean proportions of cases with a single cyst assigned to medical, percutaneous treatment, surgery and watch & wait were 52.5% (95% CI 42–65), 25.8% (95% CI 15–30), 5.1% (95% CI 0–10) and 3.3% (95% CI 0–10), respectively. 13.3% (95% CI 5–25) of cysts were staged as CL and therefore assigned to further diagnostic requirement.
Conclusion
WHO CE cyst classification and WHO-IWGE expert consensus on clinical CE management is not implemented in Mongolia. This results in exclusively surgical treatment, an unnecessary high risk approach for the majority of patients who could receive medical, percutaneous treatment or observation (watch & wait). Introduction of WHO-IWGE expert consensus and training in ultrasound CE cyst staging would be highly beneficial for patients and the health care services.
Author summary
Cystic Echinococcosis (CE) is a zoonotic disease, commonly known as dog tapeworm. The disease is distributed globally and predominantly affects rural populations with limited access to health care. Following the expert consensus of the WHO-Informal Working Group on Echinococcosis (WHO—IWGE) patients with uncomplicated cysts are assigned on the basis of WHO cyst classification to four treatment modalities: medical (benzimidazoles), percutaneous, surgical treatment, and ‘watch & wait’. In Mongolia, one third of the population practices nomadic farming. These populations are heavily affected by CE. However, cyst staging and WHO-IWGE recommendations are not implemented and patients referred to the three national treatment centres receive surgical treatment. This exposes a large proportion of patients to an unnecessary high risk approach who could be treated–depending on cyst stage—with benzimidazoles, percutaneously or observed (watch & wait). We reviewed the hospital records of patients with CE and admitted between 2008 and 2015 to the three national CE treatment centres, retrospectively staged the cysts and assigned the patients to the four WHO-IWGE recommended treatment modalities. We found a high proportion of patients in the study population who would have most likely benefitted from non-surgical treatment options.
Introduction
Cystic echinococcosis (CE) is a zoonotic disease caused by the larval stage of Echinococcus granulosus. The life cycle of E. granulosus is maintained between the dog as the definitive host and various livestock as the intermediate host. Humans are considered as an aberrant intermediate host. Ingested larvae develop into cystic lesions, mostly in the liver and lung [1]. The disease is globally distributed including Central Asian countries and China [2–4]. The annual global burden of disease is estimated at 184,000 Disability Adjusted Life Years (DALYs) [5, 6]. Due to a large proportion of asymptomatic cases and underreporting the disease burden is widely underestimated. Pastoral communities in countries with limited resources bear the greatest burden [3, 5, 7].
In Mongolia, one-third of the population is engaged in extensive pastoral farming. The presence of a large livestock population accompanied by watchdogs, a big number of stray dogs, unregulated private slaughtering, and lack of health education are the main reasons for the heavy human CE exposure [8, 9]. Historically, due to strong public and veterinary action, the surgical cases decreased from 13% in 1946 to 2% in 1988 in the state hospital [10, 11]. After the Soviet Union collapsed in 1990, the veterinary and public health sectors weakened and many control programs for zoonotic disease, including CE, collapsed [12]. Currently, CE is not included in the national surveillance system, and official statistics are, therefore, unavailable [8, 9, 13]. There are few reports on the current transmission of CE in Mongolia including small scale serological surveys, hampered, however, by the sensitivity and specificity problems of serological CE testing [11, 14–18].
CE predominantly affects rural populations with very limited access to health care [19]. Diagnosis, cyst staging, treatment and follow-up depend on imaging [20, 21]. Ultrasonography, however, is only recently introduced in low and lower middle income countries (LICs and LMICs)[22–24]. Thus most endemic countries, including Mongolia, have not yet implemented the WHO Informal Working Group on Echinococcosis (WHO-IWGE) expert consensus [25, 26]. The core piece of the WHO-IWGE expert consensus is to triage on the basis of ultrasound-defined cyst stages into four groups: medical, percutaneous, surgical treatment (active cyst stage CE1 to CE3b) and ‘watch & wait’ (inactive cyst stages CE4 and CE5) [20, 27–30].
The goal of this study is to describe the current clinical management of CE in Mongolia, to capture the distribution of cyst stages retrospectively from stored ultrasound images, to critically contrast current practice in Mongolia with WHO-IWGE expert consensus and to suggest a LIC / LMICs-adapted implementation strategy for WHO-IWGE expert consensus.
Materials and method
Ethics statement
This work presented here was approved by the Medical Ethics committee of Mongolia (July 2014) and WHO ERC (27 Nov 2015).
Study area and data collection
We reviewed the hospital records of patients diagnosed with CE and admitted between 2008 and 2015 to the three state hospitals conducting CE surgery in Mongolia: First Central Hospital (FCH), Third Central Hospital (TCH), National Center for Maternal and Child Health (NCMCH). Patients identified as probable CE cases in the peripheral or secondary hospitals are, as a rule, referred to the three state hospitals for confirmation and surgery. In the archives of the state hospitals, the medical records are chronologically stored in bundles of 150–200 reports. On the front page of each record the discharge diagnosis is recorded by the surgeon, based on histopathology which is done as a routine in the national hospitals. The ‘discharge diagnosis CE’ (ICD 10 code B.67.0–67.9) was the inclusion criteria for our study.
The following data were extracted from the patient records on data extraction sheets: demographic and geographic data (specified in Table 1), clinical symptoms and signs, ultrasonography (US) reports including number and size of cysts, US images, surgical reports and final diagnosis. US images (photos) were digitalized and stored. The data collected on data extraction sheets were double entered into a data base.
Table 1. Demography, socio-economic status (SES) and geographical data of the patients.
| Patient characteristics | Frequency | |
|---|---|---|
| n | % | |
| Age | ||
| <14 | 113 | 45.7 |
| 15–59 | 116 | 47.0 |
| >60 | 18 | 7.3 |
| Total | 247 | 100 |
| Sex | ||
| Male | 116 | 47.0 |
| Female | 131 | 53.0 |
| Total | 247 | 100 |
| Occupation | ||
| Paid employee | 42 | 34.4 |
| Self-employed | 16 | 13.1 |
| Employed in animal husbandry | 12 | 9.8 |
| Unemployed/retired/disabled | 52 | 42.6 |
| Total | 122* | 100 |
| Distance from secondary level hospital | ||
| <100 km | 107 | 62.2 |
| 100 km-200 km | 53 | 30.8 |
| >200 km | 12 | 6.9 |
| Total | 172** | 100 |
| Type of home | ||
| Apartment | 47 | 19.0 |
| Yurt | 200 | 81.0 |
| Total | 247 | 100 |
*- 60 pediatric cases and 65 cases without information on employment were excluded from calculating the percentage of occupation.
**- 60 cases from Ulaanbaatar city and 15 cases without an exact address were excluded from calculating the percentage of distance
Data analysis
The demographic information including age, sex, occupation, type of home, and distance from health care was presented with the relevant percentage. The distance between secondary hospital (provincial general hospital) and current address of the patient was calculated. The levels of the clinical care in Mongolia are provided in Fig A in S1 Appendix.
The socio-economic status (SES) in the adult patients was stratified according to the labour force category of the National Statistical Office [31]. The current address of the patient was used to plot the geographical distribution by employing the ArcGIS 10.0 (ESRI 2011. ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute). The annual surgical incidence was estimated based on the hospital records. The frequency of the clinical signs & symptoms was calculated. The size and number of CE cysts were presented in three different categories, <5 cm, 5–10 cm, and >10 cm and single cyst, 1–3 cysts, and multiple cysts, respectively.
The digital copies of US cyst images were independently reviewed by three international experts (reviewers) to estimate the frequency distribution of cyst stages according to WHO cyst classification (CL, CE1, CE2, CE3a, CE3b, CE4, CE5) in the patient population referred to the state hospitals for confirmatory assessment and treatment. The exercise was performed on the US images of abdominal lesions. The experts received no further information about the patients. Duplicate images of cysts were provided when available. If the reviewer felt unable to stage a cyst, e.g. because of poor quality of an image, the cyst was classified ‘not identifiable’ (NI). After the assessment, exclusions were made on the double images. If a double image of a cyst was assessed identical by the reviewers, we selected randomly one of the images. If a double image was assessed not identical by the reviewers, the one with fewer “NI” votes was selected to represent the cyst. Interrater-agreement was calculated using the Kappa statistic. Raw agreement was calculated as the proportion of cysts where the raters noted the same stage and kappa statistics for ordered categories (CL, CE1, CE2, CE3a, CE3b, CE4, CE5). We report both, unweighted and weighted kappa statistics with weights calculated as the square of the distance between the two ordinal groups. We judged agreement as poor if κ < 0.2; fair if 0.2 ≤ κ < 0.4; moderate if 0.4 ≤ κ < 0.6; substantial if 0.6 ≤ κ < 0.8; and good if κ ≥ 0.8. The estimates represent the arithmetic mean of the 3 pair-wise comparisons. “NI” judgements were considered as missing values which have been excluded pair-wise for raw agreement and kappa estimation.
Allocation of the cysts to WHO recommended treatment modalities was performed on the basis of cyst staging by the 3 reviewers and cyst size. Cases with images assessed as “NI” by all three reviewers were excluded; equally, cases with more than one cyst since completeness and ascertainment of US images was difficult retrospectively. The double images were excluded using the same algorithm as in the analysis of interrater-agreement. We calculated combinations of CE stage and cyst size for all three reviewer assessments separately and calculated the mean percentage. Based on this combination, the probability of assignment of cases to the treatment modalities as defined by the WHO-IWGE expert consensus were estimated for each case. Assignment of cysts to WHO recommended treatment modalities was performed as if all cysts were uncomplicated since the retrospective data did not allow to reliably differentiate complicated from uncomplicated cysts. Bootstrap resampling with 10,000 replicates was used for estimating the 95% confidence intervals for the treatment options. The analysis was conducted using the statistical package R v 3.4.0.
Results
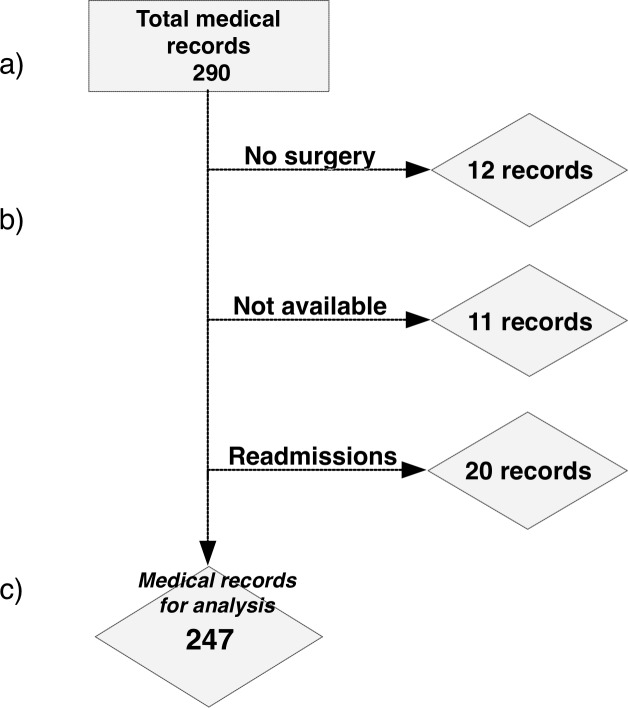
A total of 290 medical records fulfilled the inclusion criterion ‘discharge diagnosis CE’ (ICD 10 code B.67.0–67.9) of the patients admitted to the three state hospitals between 2008 and 2015, 43 records were excluded. For details see Fig 1.
Fig 1. Selection of CE surgical case records.
(a) 290 records (patients) in the 3 state hospitals in Mongolia fulfilled the inclusion criterion ‘discharge diagnosis CE’ (ICD 10 code B.67.0–67.9); (b) 12 records (patients) were excluded because the patients have not been operated on (4 surgeries postponed, 3 post-surgical conditions, 4 calcified cysts not needing surgery, 1 no information), 11 because of missing surgical information, 20 records belonged to 15 patients who were re-admitted once or twice; (c) 247 cases were analyzed.
The demographic data are presented in Table 1.
During the period 2008–2015, the average annual CE surgical incidence per 100 000 was 1.06 (95% CI 0.7–1.4) based on the current data collection. On average 30.8 (95% CI 20.4–41.3) cases per annum underwent CE surgery in the central hospitals between 2008 and 2015 (Fig B in S1 Appendix).
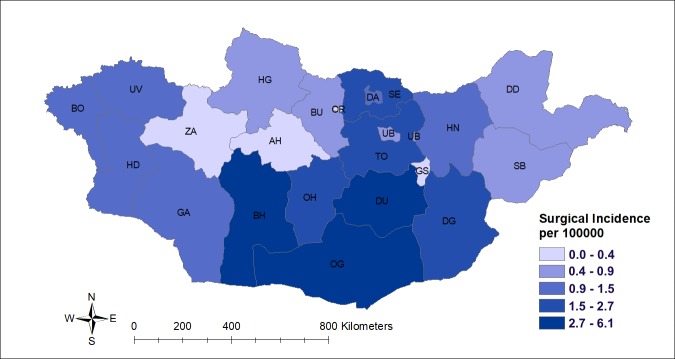
The patients originated from 20 provinces corresponding to 95% (20/21) of all provinces (Fig 2). The southern provinces Omnogobi (OG), Dundgobi (DU), and Bayankhongor (BH) have the highest number of cases. Average CE surgical incidence for the survey period in these provinces was 2.7–6.1 per 100 000 inhabitants based on the medical records in the three state hospitals.
Fig 2. Average CE surgical incidence in each province for the period 2008–2015.
Abbreviations: BO-Bayan-Olgii, UV-Uvs, HD-Khovd, ZA-Zavkhan, GA-Gobi-Altai, BH-Bayankhongor, OH-Ovorkhangai, AH- Arkhangai, HG-Khubsgul, BU-Bulgan, DA-Darkhan, SE-Selenge, ER- Orkhon, UB-Ulaanbaatar, DU-Dundgobi, OG-Omnogobi, TO- Tov, DG-Dornogobi, HN-Khentii, DD-Dornod, GS-Gobisumber., SB-Sukhbaatar.
The frequency of symptoms & signs in CE patients at admission to the three state hospitals are provided in Fig C in S1 Appendix. The location of CE cysts of the surgically treated patients at the three state hospitals between 2008 and 2015 are provided in Fig D in S1 Appendix.
Preoperative ultrasound reports were available in 83.5% (152/182) of patients who had undergone abdominal surgery for CE. 26.3% (40/152) were explicitly recorded as hydatid cysts, 54.6% (83/152) as cystic lesions, and 19% (29/152) as other space occupying lesions. 99.3% (151/152) of the abdominal cases had the information on the cyst number in the US record. Among them, 78.1% (118/151) had single cysts, 20% (31/151) had 1–3 cysts and 1.3% (2/151) had more than 3 cysts. The information on size was available for 90.1% (137/152) of the abdominal cases. Among them, 48.9% (67/137) had cysts smaller than 5 cm, 35.0% (48/137) of 5–10 cm and 16.1% (22/137) were bigger than 10 cm.
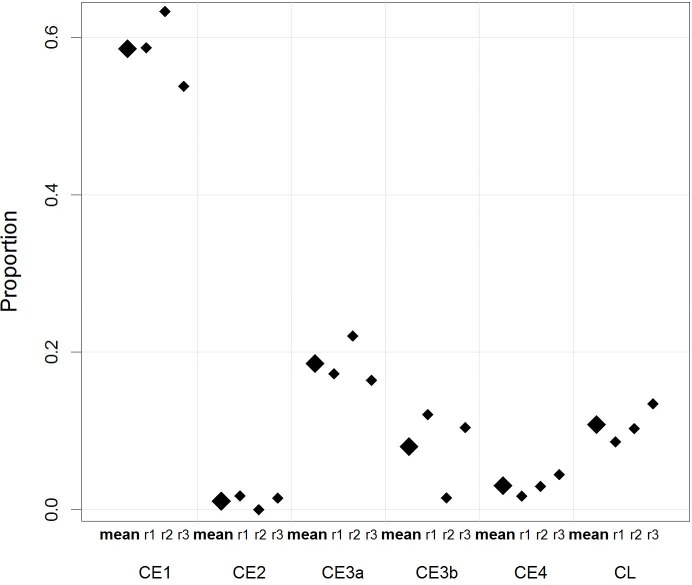
138 US imaging photos of 84 cases with liver and abdominal cysts were available for review and CE cyst staging based on the WHO CE cyst classification by three international reviewers. Following the inclusion / exclusion procedures described in the method section images of 84 unique cysts were assessed for interrater-agreement analysis. The raw agreement was 77.2%. We observed substantial agreement with an average unweighted Kappa coefficient of 0.57 and an average weighted Kappa coefficient of 0.59 (squared weights). The proportions of CE cyst stages are shown in Fig 3. Mean proportion for CE stages including CE1, CE2, CE3a, CE3b, CE4, and CL were 0.59, 0.01, 0.19, 0.08, 0.03 and 0.11, respectively. No CE5 cysts were identified.
Fig 3. Distribution of CE cyst stages on the basis of the WHO CE cyst classification.
The average proportions (big diamond) of CE cyst stages staged on the basis of the WHO CE cyst classification by the three experts. r1, r2, r3 are the proportions (small diamonds) of CE cyst stages classified by the expert 1, 2, and 3, respectively.
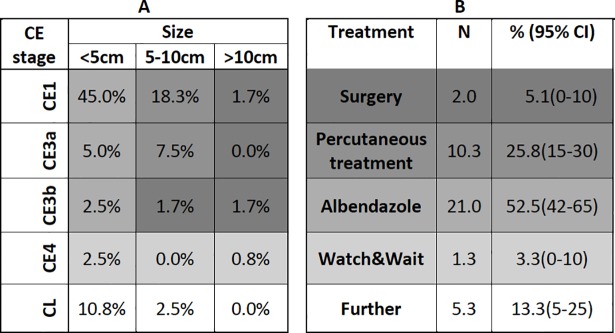
Of the cysts staged by the three reviewers, 40 met the inclusion criteria for the treatment modality analysis (selection process see methodology section). The mean proportion of cases assigned to medical treatment, percutaneous treatment, surgery and watch & wait was calculated as 52.5% (95% CI 42–65), 25.8% (95% CI 15–30), 5.1% (95% CI 0–10) and 3.3% (95% CI 0–10), respectively. 13.3% (95% CI 5–25) of the cysts were staged as CL in need for further diagnostic work up (Fig 4).
Fig 4. Assignment of CE cysts of the study population to WHO-IWGE recommended treatment modalities on the basis of retrospective cyst staging by three international experts.
In uncomplicated CE cysts WHO-IWGE recommended assignment of patients to the four treatment modalities surgery, percutaneous methods, drug treatment (albendazole, mebendazole) and watch & wait is based on cysts stage and size (three groups). A Three size groups and the average percentages of WHO cysts stages in the study population as retrospectively staged by three international reviewers. Although there was a small percentage (1%) of CE2 identified by the three reviewers (Fig 3) this stage could not be included into the analysis because the information on cyst size was lacking. CE5 was excluded since none of the reviewers attributed one of the cysts presented for retrospective staging to CE5. B Based on the matrix in A, the probability of assignment of patients to the WHO-IWGE recommended treatment modalities is presented. The grey shading of the cells in A and B matrices correspond to each other. Cysts staged as CL would have needed further diagnostic work up.
Discussion
To the best of our knowledge this is the first description of the clinical management of CE patients in LICs / LMICs with an attempt to critically contrast current national practice with the WHO-IWGE expert consensus [20] on the basis of interrater-agreement calculated from the retrospective cyst staging by three independent experts. Mongolia offers the unique opportunity for this exercise for two reasons. CE patients identified on the peripheral level of the health care services are exclusively referred for confirmation and treatment to the three state hospitals where patient records are carefully stored in archives with a discharge diagnosis on the front page of each patient file. The data of 290 patients with the ‘discharge diagnosis CE’ (ICD 10 code B.67.0–67.9) admitted between 2008 and 2015 were available to characterize the CE patients who currently receive treatment in Mongolia. Ultrasound photos of 46% (84/182) of patients with abdominal cysts were available for retrospective cyst staging on the basis of WHO CE cyst classification by three international experts. Based on the retrospective staging result and size, proportions of assignment to WHO-IWGE recommended treatment modalities were estimated.
The socio-demographic characteristics of the study population receiving treatment at the three state hospitals show some interesting features. Around 50% of the patients are below 15 years of age. In Kazakhstan and Kyrgyzstan, countries in the same region as Mongolia and with a similar pastoral lifestyle, also reported high proportion of children among the surgical cases [3]. Equal proportions of male and female patients suggest that, understandably, the infection risk is equal with CE transmitted in a food borne dog faecal-human oral cycle. Distance to the secondary hospital is a relevant factor for access to specialized health care in all LICs and most MICs [32, 33]. About 40% of the surgical patients had to travel at least 100 km to reach the secondary hospitals. Besides the geographic barrier other factors might contribute to underreporting but currently no data are available. 80% of the patients are living in a “yurt” which suggest that these patients are exposed to the combination of poor infrastructure, poor water and sanitation and frequent contact with watch-dogs and their faeces [34, 35].
A significant proportion of patients presented with non-specific symptoms, around 20% with abdominal pain corresponding to 29% of the patients with abdominal cysts. Around 16.6%, 8.5% and 6.9% of all patients had cough, chest pain and dyspnoea, respectively. This amounts to 50%, 32%, and 14%, respectively, of patients with lung cyst. These results are in line with the clinical experience that most patients present with non-specific symptoms and signs [36]. Fever, observed in 22.3% of all admissions, may, however, indicate that some patients had complication such as a cysto-biliary fistula with secondary bacterial infection of the cyst or cholangitis associated with biliary obstruction due to spillage of cyst content into the biliary tree; the latter also causes abdominal pain [37]. Similarly, patients with lung cysts may have had fever due to pneumonia following bronchial compression caused by expanding cysts or secondary bacterial infection of the residual cavity after the cyst content has been expectorated via a cysto-bronchial fistula. Both complications are associated with cough and dyspnoea [38]. Care should be exercised, however, to not over interpret retrospectively non-specific signs and symptoms such as fever, documented in medical records.
Most patients had only a single cyst in abdominal organs of which almost 50% had a diameter equal or smaller than 5 cm. This alone casts doubts on the indication for major surgery. If uncomplicated, these cysts would—following the WHO-IWGE expert consensus—either fall in the medical treatment group, if active (CE1 to CE3), or the watch & wait group, if inactive (CE4 and 5).
Only 40 reports of the 182 abdominal cysts examined by ultrasound spelled out “hydatid cyst” as the US diagnosis, without, however, mentioning accepted US criteria and without an attempt to stage with the WHO-IWGE or Gharbi classification.
The most exciting part of this data set was the unique opportunity to review and retrospectively stage 138 US imaging photos of 84 patients by three international experts to compare current practice in Mongolia with WHO recommended treatment modalities [20]. The distribution of WHO cyst stages retrospectively analysed from a subset of patients treated between 2008 and 2015 at the three state hospitals in Mongolia showed proportions of 0.59, 0.01, 0.19, 0.08, 0.03 and 0.11 for CE1, CE2, CE3a, CE3b, CE4, and CL, respectively (see Fig 3). Given the relatively high number of categories, the observed raw agreement of about 80% and Kappa coefficients of close to 0.6 indicate that the method appears to be generally valid but with room for improvement. A similar result was found in a recent study assessing interrater-agreement of the WHO cyst classification based on 2-D US images. The authors emphasized that an improvement can be achieved when non-static imaging (video recording etc.) is incorporated in future studies [39].
Assignment of patients to the WHO-IWGE recommended treatment modalities takes into account cyst size in addition to cyst stage. Merging the cyst sizes with the retrospective WHO cyst staging of the study population provides an insight into deviations of current practice in Mongolia from the WHO-IWGE expert consensus. Of particular significance for patients regarding unnecessary treatment risks and for the health care services with respect to avoidable treatment costs is the fact that 3.3% of abdominal cysts would have not warranted any treatment at all since they were already in an inactive stage (CE 4). Following the WHO-IWGE expert consensus they would have been submitted to the watch & wait approach with a very high probability of no need for further treatment [28, 29]. 52.5% of active abdominal cysts (CE1 to CE3) with a diameter of 5 cm or smaller would have been submitted to medical treatment following the WHO-IWGE expert consensus [20, 40]. This draws attention to the fact that availability of albendzole, the preferred benzimidazole for CE therapy, is hugely lacking in Mongolia. Currently, the price of albendazole is very high with approximately USD 2 per 400mg tablet. Albendazole is mostly sold in pharmacies in cities, which also limits access. Access to albendazole is a very much debated issue in the care for the neglected tropical disease echinococcosis in neglected populations [41]. In addition to albendazole cost, 10–15% of the hospital admission fee of public hospital is paid by the patients and the remaining cost is covered by the national health system in Mongolia [42]. However, there are many other costs including informal fees to clinicians and advanced diagnostics increasing the economic burden CE patients have to carry. Almost 26% of the patients assessed would have been allocated to percutaneous treatment (PT). PT options are generally underused and are not carried out in Mongolia for CE management due to a lack of trained personnel. Introducing PT into Mongolia should be considered because it carries less risks for the patients, reduces treatment cost and length of hospital stay. A study in Bulgaria showed similar deviations from best practices as recommended by WHO-IWGE expert consensus[43].
There are several limitations of the study. The data were extracted retrospectively from patient files. The fact that in Mongolia CE patients are being treated exclusively at the three state hospitals with high standards of documentation and archiving compensated partly for this shortcoming. Also, cyst staging was retrospectively performed on the basis of 2-D ultrasound photos. Our interrater-agreement of retrospective cyst staging, however, gives confidence that the staging result provides an acceptable estimate of the distribution of cyst stages seen at the three national hospitals. Identifying complicated cysts is difficult in retrospective studies. In some instances we could suspect that a cyst was complicated by combining various pieces of available retrospective information (e.g. fever, abdominal pain plus US cyst features suspicious of secondary bacterial infection of a cyst). This, however, is all too vague and would be an over interpretation of retrospective data. We thus did the analysis “as if” the cysts of the US images classified were uncomplicated. This may have resulted in an overestimate of the non-surgical treatment modalities.
In conclusion, our study demonstrates
Mongolia has a significant section of the population suffering from cystic echinococcosis and is in need of implementing a LMIC-adapted version of the WHO-IWGE expert consensus
Access to the privileged treatment centres, the three state hospitals, may be limited by geographic distance and economic resources
Ultrasonography is available, but diagnostic CE criteria seem not to be sufficiently well known and cyst staging is not performed
WHO-IWGE expert consensus for assigning patients to the four treatment options based on CE cyst staging and cyst size are not implemented resulting in surgical treatment of all CE patients and in an unnecessary high risk approach in patients who could be treated with albendazole or percutaneously or observed (watch & wait).
It is recommended
To systematically include CE into the disease surveillance system of Mongolia
To make ultrasonography available to the primary / secondary health care level and to train local, physicians in the WHO CE cyst staging. The Focused Assessment with Sonography for Echinococcosis (FASE), a short course for general practitioners, could be a model [26]. In 2016, a pilot training was conducted on WHO-CE cyst classification and staging for primary/secondary ultrasonography doctors by leading experts in the field.
To introduce and implement the WHO-IWGE expert consensus at all levels of the health care system to triage patients into the four WHO treatment modalities (a) medical treatment of small active cysts, (b) percutaneous treatment of larger active cysts (CE 1 and CE 3a), (c) surgery for complicated, very large cysts and cysts unresponsive to medical therapy, and (d) watch & wait for inactive cysts (CE4 and 5). Implementation at the primary and secondary health care levels saves transport cost, travel time for patients and allows a substantial proportion of patients to stay near or in their homes during treatment.
To apply our strategy of assessing CE clinical epidemiology and management in other CE endemic LICs / LMICs as a basis for planning.
Supporting information
(PDF)
Acknowledgments
The authors are greatly thankful to Dr Francesca Tamarozzi, from University of Pavia and Dr Marija Stojković from Heidelberg University Hospital for reviewing the ultrasonographic images for CE staging.
We thank Dr Enkhtur Shonkhuuz from National Center for Maternal and Child Health of Mongolia for giving the permission to review medical records.
We would also like to express our gratitude to Dr Bernadette Abela-Ridder from Neglected Zoonotic Diseases in Department of the Control of Neglected Tropical Diseases, WHO, Geneva for her continuous support, technical guidance and encouragement.
Data Availability
Data are available from the National Center for Zoonotic Disease of Mongolia (NCZD) for researchers who meet the criteria for access to confidential data. The point of contact email for the National Center for Zoonotic Disease of Mongolia - Tsogbadrakh Nyamdorj, director. email: tsogbadrakh@nczd.gov.mn.
Funding Statement
This investigation received financial support from the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (WHO/TDR), Impact Grant 2014-B4013, http://www.who.int/tdr/news/2015/impact-grant/en/. Initial part of data collection received financial support from the World Bank/Capacity Building for Emerging Infectious Disease Preparedness Project which implemented in Mongolia (project ID: P131204), http://projects.worldbank.org/P131204/capacity-builiding-emerging-infectious-disease-preparedness?lang=en&tab=overview. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, et al. Ecology and Life Cycle Patterns of Echinococcus Species. Adv Parasitol. 2017;95:213–314. 10.1016/bs.apar.2016.11.002 . [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Zhang Z, Wu W, Shi B, Li J, Zhou X, et al. Epidemiology and control of echinococcosis in central Asia, with particular reference to the People's Republic of China. Acta Trop. 2014. 10.1016/j.actatropica.2014.03.014 . [DOI] [PubMed] [Google Scholar]
- 3.Torgerson PR, Oguljahan B, Muminov AE, Karaeva RR, Kuttubaev OT, Aminjanov M, et al. Present situation of cystic echinococcosis in Central Asia. Parasitol Int. 2006;55 Suppl:S207–12. 10.1016/j.parint.2005.11.032 . [DOI] [PubMed] [Google Scholar]
- 4.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv Parasitol. 2017;95:315–493. 10.1016/bs.apar.2016.11.001 . [DOI] [PubMed] [Google Scholar]
- 5.Budke CM, Jiamin Q, Zinsstag J, Qian W, Torgerson PR. Use of disability adjusted life years in the estimation of the disease burden of echinococcosis for a high endemic region of the Tibetan plateau. Am J Trop Med Hyg. 2004;71(1):56–64. . [PubMed] [Google Scholar]
- 6.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12(12):e1001920 10.1371/journal.pmed.1001920 ; PubMed Central PMCID: PMCPMC4668834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devleesschauwer B, Ale A, Torgerson P, Praet N, Maertens de Noordhout C, Pandey BD, et al. The burden of parasitic zoonoses in Nepal: a systematic review. PLoS neglected tropical diseases. 2014;8(1):e2634 10.1371/journal.pntd.0002634 ; PubMed Central PMCID: PMC3879239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurbadam A, Nyamkhuu D, Nyamkhuu G, Tsendjav A, Sergelen O, Narantuya B, et al. Mongolian and Japanese Joint Conference on "Echinococcosis: diagnosis, treatment and prevention in Mongolia" June 4, 2009. Parasit Vectors. 2010;3(1):8 10.1186/1756-3305-3-8 ; PubMed Central PMCID: PMCPMC2829607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFadden AM, Muellner P, Baljinnyam Z, Vink D, Wilson N. Use of Multicriteria Risk Ranking of Zoonotic Diseases in a Developing Country: Case Study of Mongolia. Zoonoses Public Health. 2016;63(2):138–51. 10.1111/zph.12214 . [DOI] [PubMed] [Google Scholar]
- 10.Davaatseren N, Otogondalai A, Nyamkhuu G, Rusher AH. Management of echinococcosis in Mongolia. J Ark Med Soc. 1995;92(3):122–4. . [PubMed] [Google Scholar]
- 11.Ito A, Budke CM. The present situation of echinococcoses in Mongolia. J Helminthol. 2015;89(6):680–8. 10.1017/S0022149X15000620 . [DOI] [PubMed] [Google Scholar]
- 12.Ebright JR, Altantsetseg T, Oyungerel R. Emerging infectious diseases in Mongolia. Emerg Infect Dis. 2003;9(12):1509–15. 10.3201/eid0912.020520 ; PubMed Central PMCID: PMCPMC3034321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinchuluun B, Sako Y, Khatanbaatar I, Bayarmaa B, Lkhagvatseren S, Battsetseg G, et al. A survey of seropositivity to antigen B, an immunodiagnostic antigen for human cystic echinococcosis, in domestic animals in Mongolia. Parasitol Int. 2014;63(2):324–6. 10.1016/j.parint.2013.12.002 . [DOI] [PubMed] [Google Scholar]
- 14.Ito A, Dorjsuren T, Davaasuren A, Yanagida T, Sako Y, Nakaya K, et al. Cystic echinococcoses in Mongolia: molecular identification, serology and risk factors. PLoS Negl Trop Dis. 2014;8(6):e2937 10.1371/journal.pntd.0002937 ; PubMed Central PMCID: PMCPMC4063745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabbar A, Narankhajid M, Nolan MJ, Jex AR, Campbell BE, Gasser RB. A first insight into the genotypes of Echinococcus granulosus from humans in Mongolia. Mol Cell Probes. 2011;25(1):49–54. 10.1016/j.mcp.2010.11.001 . [DOI] [PubMed] [Google Scholar]
- 16.Ito A, Agvaandaram G, Bat-Ochir OE, Chuluunbaatar B, Gonchigsenghe N, Yanagida T, et al. Histopathological, serological, and molecular confirmation of indigenous alveolar echinococcosis cases in Mongolia. Am J Trop Med Hyg. 2010;82(2):266–9. 10.4269/ajtmh.2010.09-0520 ; PubMed Central PMCID: PMCPMC2813168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh S, Yu JR, Kim JI, Gotov C, Janchiv R, Seo JS. Intestinal protozoan infections and echinococcosis in the inhabitants of Dornod and Selenge, Mongolia (2003). Korean J Parasitol. 2006;44(2):171–4. 10.3347/kjp.2006.44.2.171 ; PubMed Central PMCID: PMCPMC2532637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, He T, Wen X, Li T, Waili TT, Zhang W, et al. Human cystic echinococcosis in two Mongolian communities in Hobukesar (China) and Bulgan (Mongolia). Trans R Soc Trop Med Hyg. 2005;99(9):692–8. 10.1016/j.trstmh.2005.01.004 . [DOI] [PubMed] [Google Scholar]
- 19.Budke CM, Jiamin Q, Qian W, Torgerson PR. Economic effects of echinococcosis in a disease-endemic region of the Tibetan Plateau. Am J Trop Med Hyg. 2005;73(1):2–10. . [PubMed] [Google Scholar]
- 20.Brunetti E, Kern P, Vuitton DA, Writing Panel for the W-I. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010;114(1):1–16. 10.1016/j.actatropica.2009.11.001 . [DOI] [PubMed] [Google Scholar]
- 21.Stojkovic M, Rosenberger K, Kauczor HU, Junghanss T, Hosch W. Diagnosing and staging of cystic echinococcosis: how do CT and MRI perform in comparison to ultrasound? PLoS Negl Trop Dis. 2012;6(10):e1880 10.1371/journal.pntd.0001880 ; PubMed Central PMCID: PMCPMC3493391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belard S, Tamarozzi F, Bustinduy AL, Wallrauch C, Grobusch MP, Kuhn W, et al. Point-of-Care Ultrasound Assessment of Tropical Infectious Diseases—A Review of Applications and Perspectives. Am J Trop Med Hyg. 2016;94(1):8–21. 10.4269/ajtmh.15-0421 ; PubMed Central PMCID: PMCPMC4710450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker DM, Tafoya CA, Becker SL, Kruger GH, Tafoya MJ, Becker TK. The use of portable ultrasound devices in low- and middle-income countries: a systematic review of the literature. Trop Med Int Health. 2016;21(3):294–311. 10.1111/tmi.12657 . [DOI] [PubMed] [Google Scholar]
- 24.Groen RS, Leow JJ, Sadasivam V, Kushner AL. Review: indications for ultrasound use in low- and middle-income countries. Trop Med Int Health. 2011;16(12):1525–35. 10.1111/j.1365-3156.2011.02868.x . [DOI] [PubMed] [Google Scholar]
- 25.Nabarro LE, Amin Z, Chiodini PL. Current management of cystic echinococcosis: a survey of specialist practice. Clin Infect Dis. 2015;60(5):721–8. 10.1093/cid/ciu931 . [DOI] [PubMed] [Google Scholar]
- 26.Del Carpio M, Mercapide CH, Salvitti JC, Uchiumi L, Sustercic J, Panomarenko H, et al. Early diagnosis, treatment and follow-up of cystic echinococcosis in remote rural areas in Patagonia: impact of ultrasound training of non-specialists. PLoS Negl Trop Dis. 2012;6(1):e1444 10.1371/journal.pntd.0001444 ; PubMed Central PMCID: PMCPMC3254659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junghanss T, da Silva AM, Horton J, Chiodini PL, Brunetti E. Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg. 2008;79(3):301–11. . [PubMed] [Google Scholar]
- 28.Piccoli L, Tamarozzi F, Cattaneo F, Mariconti M, Filice C, Bruno A, et al. Long-term sonographic and serological follow-up of inactive echinococcal cysts of the liver: hints for a "watch-and-wait" approach. PLoS Negl Trop Dis. 2014;8(8):e3057 10.1371/journal.pntd.0003057 ; PubMed Central PMCID: PMCPMC4133254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stojkovic M, Rosenberger KD, Steudle F, Junghanss T. Watch and Wait Management of Inactive Cystic Echinococcosis—Does the Path to Inactivity Matter—Analysis of a Prospective Patient Cohort. PLoS Negl Trop Dis. 2016;10(12):e0005243 10.1371/journal.pntd.0005243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stojković M, Gottstein B, Junghanss T. Echinococcosis 2014. In: Manson's Tropical Diseases [Internet]. Saunders Ltd; 23. [Google Scholar]
- 31.NSO [updated 2017]. Available from: nso.mn.
- 32.Bitton A, Ratcliffe HL, Veillard JH, Kress DH, Barkley S, Kimball M, et al. Primary Health Care as a Foundation for Strengthening Health Systems in Low- and Middle-Income Countries. J Gen Intern Med. 2017;32(5):566–71. 10.1007/s11606-016-3898-5 ; PubMed Central PMCID: PMCPMC5400754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills A. Health care systems in low- and middle-income countries. N Engl J Med. 2014;370(6):552–7. 10.1056/NEJMra1110897 . [DOI] [PubMed] [Google Scholar]
- 34.Narrod C, Zinsstag J, Tiongco M. A one health framework for estimating the economic costs of zoonotic diseases on society. Ecohealth. 2012;9(2):150–62. 10.1007/s10393-012-0747-9 ; PubMed Central PMCID: PMCPMC3415616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Possenti A, Manzano-Roman R, Sanchez-Ovejero C, Boufana B, La Torre G, Siles-Lucas M, et al. Potential Risk Factors Associated with Human Cystic Echinococcosis: Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2016;10(11):e0005114 10.1371/journal.pntd.0005114 ; PubMed Central PMCID: PMCPMC5098738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Budke CM, Carabin H, Ndimubanzi PC, Nguyen H, Rainwater E, Dickey M, et al. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am J Trop Med Hyg. 2013;88(6):1011–27. 10.4269/ajtmh.12-0692 ; PubMed Central PMCID: PMCPMC3752796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Symeonidis N, Pavlidis T, Baltatzis M, Ballas K, Psarras K, Marakis G, et al. Complicated liver echinococcosis: 30 years of experience from an endemic area. Scand J Surg. 2013;102(3):171–7. 10.1177/1457496913491877 . [DOI] [PubMed] [Google Scholar]
- 38.Hozakova L, Roznovsky L, Mittak M, Bartek T, Chmelova J, Dvorackova J, et al. [Bronchobiliary fistulae as a complication of hepatic cystic echinococcosis]. Klin Mikrobiol Infekc Lek. 2011;17(2):67–70. . [PubMed] [Google Scholar]
- 39.Solomon N, Fields PJ, Tamarozzi F, Brunetti E, Macpherson CNL. Expert Reliability for the World Health Organization Standardized Ultrasound Classification of Cystic Echinococcosis. Am J Trop Med Hyg. 2017;96(3):686–91. 10.4269/ajtmh.16-0659 ; PubMed Central PMCID: PMCPMC5361546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, et al. Treatment response of cystic echinococcosis to benzimidazoles: a systematic review. PLoS Negl Trop Dis. 2009;3(9):e524 10.1371/journal.pntd.0000524 ; PubMed Central PMCID: PMCPMC2745697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamarozzi F, Nicoletti GJ, Neumayr A, Brunetti E. Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: a systematic review. Curr Opin Infect Dis. 2014;27(5):425–31. 10.1097/QCO.0000000000000093 . [DOI] [PubMed] [Google Scholar]
- 42.Tsilaanjav T, Ser-Od E, Baasai B, Byambaa G, Shagdarsuren O, Kwon S, et al. Mongolia health system review. Health Systems in Transition. 2013;3(2). [Google Scholar]
- 43.Muhtarov M, Rainova I, Tamarozzi F. Treatment of Hepatic Cystic Echinococcosis in Patients from the Southeastern Rhodope Region of Bulgaria in 2004–2013: Comparison of Current Practices with Expert Recommendations. Am J Trop Med Hyg. 2016;94(4):900–5. 10.4269/ajtmh.15-0620 ; PubMed Central PMCID: PMCPMC4824237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Data are available from the National Center for Zoonotic Disease of Mongolia (NCZD) for researchers who meet the criteria for access to confidential data. The point of contact email for the National Center for Zoonotic Disease of Mongolia - Tsogbadrakh Nyamdorj, director. email: tsogbadrakh@nczd.gov.mn.