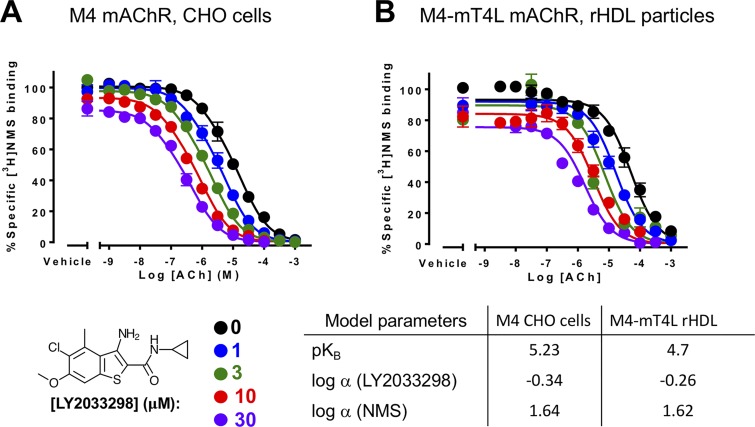
Figure 9.
Positive allosteric modulation of monomeric M4 mAChRs. (A and B) [3H]NMS interaction binding studies between the PAM, LY2033298, and acetylcholine at the M4 mAChR expressed in either CHO cells (A; replotted from Leach et al., 2010) or purified from Sf9 cells (B; M4R-mT4L; for methods, see Thal et al., 2016) and reconstituted into rHDL particles, as was previously done for rhodopsin (Whorton et al., 2007). Nearly identical levels of cooperativity were observed, indicating that LY2033298 is able to influence the binding of acetylcholine in a monomeric mAChR and is not dependent on oligomerization status of the receptor. Data from B represent the mean ± SEM of n = 3 experiments performed in duplicate.