Abstract
OBJECTIVES:
Diabetes mellitus (DM) is a common complication of chronic pancreatitis (CP). Past studies for DM risk factors in CP have been limited to single centers or highly focused on a single etiology such as alcoholic or hereditary disease. We studied risk factors for DM in a large population of patients with CP of all etiologies enrolled in the North American Pancreatitis 2 studies.
METHODS:
Participants (1,171) with CP (n =383 with DM, n =788 without DM) were enrolled prospectively from 26 participating centers. Questionnaires were completed by patients and physicians in a cross-sectional assessment. Patient demographics and disease characteristics were compared for CP with DM vs. without DM. Logistic regression was performed to assess the variables associated with DM diagnosis in a multivariable model.
RESULTS:
Diabetics were more likely to be black (P =0.02), overweight, or obese (P <0.001), and with a family history of DM (P =0.0005). CP patients with DM were more likely to have pancreatic calcifications (63% vs. 54%, P =0.002), atrophy (44% vs. 32%, P <0.0001), and prior pancreas surgery (26.9% vs. 16.9%, P <0.0001). In multivariate logistic regression modeling, the strongest risk factors for DM were obesity (odds ratio (OR) 2.8, 95% confidence interval (CI) 1.9, 4.2) and exocrine insufficiency (OR 2.4, 95% CI 1.8, 3.2).
CONCLUSIONS:
In this large multicenter cohort of patients with CP, exocrine insufficiency, calcifications, and pancreas surgery conveyed higher odds of having DM. However, the traditional ‘type 2 DM’ risk factors of obesity and family history were similarly important in conveying risk for DM.
INTRODUCTION
Although the clinical hallmark of chronic pancreatitis (CP) is recurrent and oft en severe, abdominal pain, the pathologic hallmark, is progressive fibrotic destruction of the pancreatic parenchyma (1). This progressive fibrosis leads to reduced beta cell mass, reduced insulin secretion, and eventually may result in the clinical diagnosis of diabetes mellitus (DM) (2–4). In the general US population, the prevalence of diabetes in adults is 9.3%, with the lowest prevalence in young adults and the greatest disease burden in the elderly (5). However, the risk for DM in those who have CP is much higher than the general population. The prevalence of DM in CP has been estimated anywhere between 25 and 80% (6). Increasing age, pancreatic calcifications, and prior pancreatic surgery have emerged as factors potentially conveying a higher risk for DM (7–10). However, most studies have been small or enroll heavily from a selective CP population such as alcoholic pancreatitis (7) or hereditary pancreatitis (8, 9). Although some features of pancreatitis and treatments have been considered, standard diabetes risk factors such as obesity and family history of diabetes are largely unexplored.
For the first time, we assessed both patient and disease characteristics associated with DM in a diverse cohort of adults with CP of all etiologies enrolled in the multi-center North American Pancreatitis 2 (NAPS2) studies, to determine which risk factors are associated with higher risk of DM. Expanding on existing studies, we added traditional diabetes risk factors such as body mass index (BMI) and family history to the analysis, incorporating both traditional risk factors for insulin resistance and pancreatogenic diabetes.
METHODS
NAPS2 cohort
Patients (n =1,171) with CP were prospectively enrolled in this cross-sectional study at 26 US Centers participating in the NAPS2 Program, consisting of the original NAPS2, NAPS2 continuation and validation (NAPS2-CV), and NAPS2 Ancillary studies, between 2000 and 2014. Th e NAPS group conducted three sequential studies (NAPS 2, NAPS2-CV, NAPS2 Ancillary study) to prospectively ascertain patients with recurrent acute pancreatitis, CP, and controls (related, family, and unrelated) subjects with an overarching goal to understand the role of genetic and environmental factors in the susceptibility and progression of pancreatitis. NAPS2 represents the original study cohort and NAPS2-CV the validation cohort. As the proportion of blacks in these two studies were few (7% and 8%, respectively), the NAPS2 AS study was undertaken to specifically recruit African American subjects. Detailed methodology of the NAPS2 studies has been published (11–13).
Patients were enrolled if they had CP, defined as previously described based on the following: the presence of characteristic changes on abdominal imaging studies (computerized tomography scan, magnetic resonance imaging/magnetic resonance cholangiopancreatography, endoscopic retrograde cholangiopancreatography (Cambridge classification), or endoscopic ultrasound (the presence of ≥5 findings or the presence of calcifications)) or histology. Controls and patients with recurrent acute pancreatitis but without definitive morphologic changes of CP enrolled in the NAPS2 studies were not considered for inclusion in this analysis. The study protocol was reviewed and approved by the Institutional Review Boards of all participating centers. Informed consent was obtained from participants.
Data collection
Detailed methodology and data collection procedures for the NAPS2 studies have been previously described (11–13) and included cross-sectional collection of comprehensive health questionnaires obtained from both the participant and the treating gastrointestinal specialist. Relevant portions of the questionnaires are provided in Supplementary Information S1 online. The participant questionnaire focused on collection of demographics, personal and family history, and environmental exposures. The physician component included sections on acute and CP history, including pancreatitis etiology, medical and surgical therapies, and other pertinent medical history. Questionnaires were completed by patients with assistance of a trained research coordinator and physicians in a cross-sectional assessment. Parameters assessed for this analysis included: patient demographics, social history including smoking and alcohol use, pain experience, disability or unemployment from pancreatitis-related pain, family history including diabetes in first- and second-degree relatives, physician-defined etiology of CP, treatments administered for CP, the presence of diabetes, age at onset of diabetes, if known, and medications taken for diabetes where applicable. Diabetes was stratified based on time of diagnosis as pre-existing diabetes when diabetes was diagnosed >2 years before CP onset, concurrent diabetes when diabetes was diagnosed within 2 years before CP diagnosis, and diabetes aft er CP when the diagnosis of diabetes was made aft er the CP diagnosis. Exocrine insuffi ciency was defined based on a physician-reported diagnosis of pancreatic exocrine insuffi ciency. Patients were classified as having diabetes or not having diabetes, for the purposes of this study based on the physician questionnaire response. Information on patients’ self-reported pain experience in the year preceding study enrollment is presented only from the NAPS2-CV and NAPS2 Ancillary studies. The reason for this choice was the lack of a leading question on the presence of pain (yes/no) before choosing the severity or temporal nature of pain experience in the original NAPS2 study. Data on the type and temporal nature of pain medication use was also limited to the NAPS2-CV and NAPS2 Ancillary studies, where physicians were specifically asked to provide this information.
Statistical analyses
Patient demographics and disease characteristics were compared for CP patients with diabetes and without diabetes using a t-test for continuous variables or a Fisher’s test for categorical variables. Logistic regression was performed to assess the variables associated with diabetes diagnosis in a multivariable model. Backwards model selection was used. Demographic and morphological variables that were significant in the univariable analysis were included and removed one at a time according to their significance level (Wald’s test) until a final model was reached with only significant variables remaining. Gender was borderline signifi-cant in the final model and was included. Odds ratios (ORs) and 95% confidence intervals (CIs) were generated for factors included in the final model. An interaction term between BMI and exocrine insuffi ciency was added to the final model, to test whether the relation between BMI and DM differed based on exocrine insuffi ciency. The final logistic regression model included 1,076 of the 1,171 with complete data for all variables of interest. A sensitivity analysis was performed by excluding 37 patients with a discrepant diabetes status based on the patient and physician questionnaires (patient reported diabetes but physician reported no diabetes). Two subset analyses were performed using the variables of the final logistic model. The first included only those DM patients with DM diagnosis prior to CP diagnosis. The variable duration of CP was excluded from this model, as DM diagnosis occurred before CP diagnosis. The second included DM patients with DM diagnosis after CP diagnosis. Data analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Diabetes prevalence, demographics, and treatment
Among 1171 patients in the NAPS2 patient database with physician ascertainment of current diabetes status, 383 participants had a diagnosis of DM (33%), whereas 788 (67%) were not diabetic. Participants with diabetes were older and had an older age at pancreatitis onset (age at first attack of acute pancreatitis, CP symptoms, or diagnosis, whichever is earlier), longer duration of disease, higher BMI, and lower physical component summary score on the Short Form-12 (Table 1, P<0.007 for all). Diabetics were also more likely to be of black race(P=0.02), to be overweight or obese by BMI (P<0.0001), and to have a family history of diabetes in a first-degree relative (P=0.0005). Of those participants with diabetes, 248 (65%) were treated with insulin, 136 (36%) were treated with oral anti-diabetic agents (oral agents alone in 97, both oral agents and insulin in 39), and 38 (9%) were untreated with pharmacologic therapies.
Table 1.
Demographic characteristics of NAPS2 participants with or without a diagnosis of diabetes
| No diabetes (n=788) | Diagnosed with diabetes (n=383) | ||||
|---|---|---|---|---|---|
| Patient and disease characteristics | Median or n | IQR or % | Median or n | IQR or % | P-value |
| Age at enrollment (years) | 50.44 | (39.46, 58.98) | 54.76 | (45.68, 63.55) | <0.0001 |
| Age at CP diagnosis (years) | 46 | (36, 56) | 50 | (41, 60) | <0.0001 |
| Age at first onset of pancreatitis symptoms (years) | 43 | (31, 54) | 46 | (34, 57) | 0.007 |
| Duration of disease (years) | 4.12 | (1.95, 9.44) | 5.72 | (2.33, 12.03) | 0.0001 |
| Current BMI (kg m−2) | 23.33 | (20.67, 26.54) | 25.23 | (21.7, 29.29) | <0.0001 |
| BMI category | <0.0001 | ||||
| Overweight (25–29.9 kg m−2) | 194 | 24.6% | 115 | 30.0% | |
| Obese (≥30 kg m−2) | 94 | 11.9% | 83 | 21.7% | |
| Maximum lifetime BMI (kg m−2) | 27.25 | (24, 31.19) | 31.10 | (27.46, 35.15) | <0.0001 |
| SF-12 PCS score | 36.65 | (28.75, 47.37) | 34.32 | (25.8, 45.12) | 0.0077 |
| SF-12 MCS score | 43.93 | (33.82, 53.21) | 42.97 | (33.61, 52.02) | 0.3999 |
| Gender | 0.009 | ||||
| Male | 408 | 51.8% | 230 | 60.1% | |
| Female | 380 | 48.2% | 153 | 39.9% | |
| Race | 0.02 | ||||
| Black | 148 | 18.8% | 99 | 25.9% | |
| Other | 25 | 3.2% | 9 | 2.4% | |
| White | 614 | 77.9% | 275 | 71.8% | |
| Smoking status | 0.0087 | ||||
| Never | 209 | 26.5% | 85 | 22.2% | |
| Past | 177 | 22.5% | 118 | 30.8% | |
| Current | 397 | 50.4% | 180 | 47.0% | |
| Drinking categorya | 0.0174 | ||||
| Abstainer | 138 | 17.5% | 71 | 18.5% | |
| Light to moderate use | 285 | 36.2% | 104 | 27.2% | |
| Heavy to very heavy use | 352 | 44.7% | 192 | 50.1% | |
| Family history of diabetes | |||||
| First-degree relative | 258 | 32.7% | 166 | 43.3% | 0.0005 |
| First- or second-degree relative | 416 | 52.8% | 237 | 61.9% | 0.0039 |
BMI, body mass index; CP, chronic pancreatitis; IQR, interquartile range; MCS, mental component summary; NAPS2, North American Pancreatitis study 2; PCS, physical component summary score; SF-12, Short Form −12.
Based on self-reported alcohol consumption during the maximum drinking period of life. Abstainer: no alcohol use or <20 drinks in a lifetime; light drinker: ≤3 drinks per week; moderate drinker: 4–7 drinks per week for females and 4–14 drinks per week for males; heavy drinker: 8–34 drinks per week for females and 15–34 drinks per week for males; very heavy drinker: ≥35 drinks per week for both sexes).
The prevalence of diabetes in our cohort increased with increasing age at enrollment (Figure 1). Patients with diabetes had a median age of 49 (37, 56) years at the time of diabetes diagnosis. Although most were diagnosed in adulthood, 11 patients (0.9% of cohort) had childhood-onset diabetes (age of onset <20 years, range 9–16 years), of whom 3 had CP before diabetes onset and the remaining 8 presented with CP symptoms later.
Figure 1.
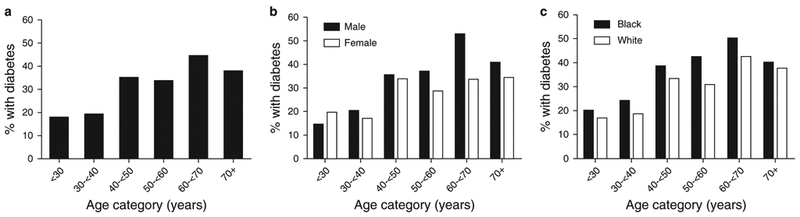
Prevalence of diabetes by age of patient at time of study enrollment (a) and subcategorized by gender (b) and by race (Black or White race, c).
When duration between pancreatitis diagnosis and diabetes onset were known(n =221), 63 participants (29%) had CP diagnosed before the diagnosis of diabetes, 67 (30%) had diabetes aft er CP was diagnosed, and 91 (41%) were diagnosed with diabetes and CP concurrently. For those participants with pancreatitis diagnosis before diabetes diagnosis, pancreatitis diagnosis preceded diabetes by a median of 3.0 (1.0, 7.0) years.
Disease characteristics, diabetes status, and therapies
Participants with diabetes were more likely to have pancreatitis attributed to hyperlipidemia (3.4% vs. 0.4%, P<0.0001) as an etiology by the enrolling physician (Table 2). However, other etiologies of CP did not differ between diabetic CP and non-diabetic CP patients. With regards to pancreas morphology, the following imaging features were reported more frequently in diabetics vs. non-diabetics: calcifications (63% vs. 54%, P =0.002), atrophy (44% vs. 32%, P <0.0001), and although pancreaticduct and common bile duct strictures and dilatation did not differ by diabetes status. Pancreatic exocrine insufficiency was more common when diabetes was also present (52% of diabetics and 30% of non-diabetics had exocrine insufficiency, P <0.0001). Exocrine insufficiency conveyed an elevated risk for diabetes across all categories of BMI (Figure 2).
Table 2.
Physician-defined etiology of chronic pancreatitis in diabetics and non-diabetic patients
| No diabetes (n=788) | Diagnosed with diabetes (n=383) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P-value | |
| Alcohol | 379 | 48.1% | 196 | 51.1% | 0.35 |
| Genetic | 70 | 8.9% | 28 | 7.3% | 0.43 |
| Idiopathic | 205 | 26.0% | 81 | 21.2% | 0.07 |
| Obstructive | 61 | 7.7% | 20 | 5.2% | 0.14 |
| Autoimmune | 15 | 1.9% | 10 | 2.6% | 0.52 |
| Hyperlipidemia | 3 | 0.4% | 13 | 3.4% | <0.0001 |
| Other | 55 | 7.0% | 35 | 9.1% | 0.20 |
Figure 2.
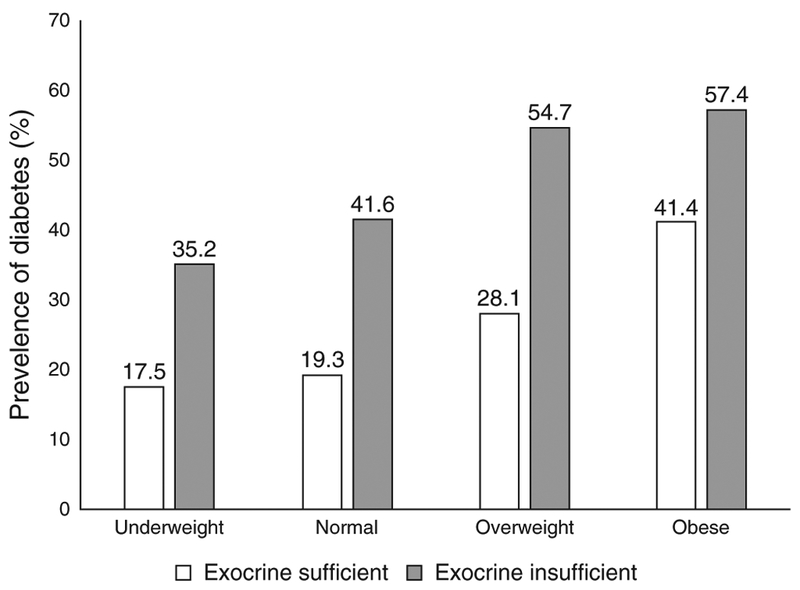
Prevalence of diabetes by body mass index (BMI) category (at study enrollment) and the presence or absence of exocrine insufficiency. Percentage of participants with diabetes is shown in the figure, from data from 117 underweight (n =54 exocrine insufficient), 556 normal weight(n =209 exocrine insufficient), 309 overweight (n =106 exocrine insufficient), and 177 (n =61 exocrine insufficient).
Those participants with diabetes were similar to non-diabetics in pain character (intermittent/chronic), pain severity, and need for opioid analgesics. Pancreatic ductal stent placement was less common in diabetic patients (P =0.003), whereas pancreatic surgery was more common in the patients with diabetes (26.9% of diabetics had prior pancreas surgery vs. 16.9% of non-diabetics, P <0.0001); pancreas resection procedures were over twice as common in the DM vs. no DM group, whereas drainage procedures were increased 1.5-fold in the DM group (Table 3). Cholecystectomy was similar between groups.
Table 3.
Treatment modalities in non-diabetic and diabetic patients with chronic pancreatitis
| No diabetes (n=788) | Diagnosed with diabetes (n=383) | ||||
|---|---|---|---|---|---|
| N | % | N | % | P-value | |
| Pain medicationsa | |||||
| Continuous opioids | 161 | 39.8% | 85 | 34.8% | 0.24 |
| Intermittent opioids | 103 | 25.4% | 57 | 23.4% | 0.5739 |
| Non-opioids only | 28 | 6.9% | 22 | 9.0% | 0.2145 |
| No pain medications | 113 | 27.9% | 80 | 32.8% | 0.3629 |
| On pancreatic enzymes | 515 | 65.4% | 267 | 69.7% | 0.1461 |
| Antioxidants or vitamins | 151 | 19.2% | 86 | 22.5% | 0.0051 |
| Celiac plexus block | 46 | 5.8% | 16 | 4.2% | 0.2672 |
| Endoscopy performed | 0.1967 | ||||
| No ERCP | 382 | 48.5% | 202 | 52.7% | |
| Other ERCP | 98 | 12.4% | 52 | 13.6% | |
| Pancreatic ERCP | 308 | 39.1% | 129 | 33.7% | |
| ERCP therapies | 406 | 51.5% | 181 | 30.8% | 0.1908 |
| Biliary stent | 88 | 11.2% | 52 | 13.6% | 0.2496 |
| Pancreatic duct stent | 269 | 34.1% | 98 | 25.6% | 0.0031 |
| Pancreatic stone removal | 95 | 12.1% | 54 | 14.1% | 0.3501 |
| Biliary or pancreatic | 321 | 40.7% | 141 | 36.8% | 0.203 |
| Sphincterotomy | |||||
| Surgery performed | 133 | 16.9% | 103 | 26.9% | <0.0001 |
| Resection procedure | 64 | 8.1% | 66 | 17.2% | <0.0001 |
| Drainage procedure | 49 | 6.2% | 38 | 9.9% | 0.0319 |
| Operation for cyst/pseudocyst | 37 | 4.7% | 32 | 8.4% | 0.0167 |
ERCP, endoscopic retrograde cholangiopancreatography; NAPS2-AS, North American Pancreatitis study 2 Ancillary study; NAPS2-CV, North American Pancreatitis study 2 continuation and validation study.
Information on pain experience and pain medication use per physician report is shown from the NAPS2-CV and NAPS2-AS studies only—relevant sample sizes were—diabetics (n =244) and non-diabetics (n =405).
The proportion of patients who reported disability or unemployment due to pain from pancreatitis was high and similar in both groups: 27% of diabetic CP and 24% of non-diabetic CP participants reported having disability/unemployment (P =0.28).
Multivariable regression analysis risk factors conveying increased OR for diabetes
Logistic regression modeling was performed to assess variables associated with diabetes diagnosis. The ORs obtained for risk of diabetes in CP patients for significant risk factors is summarized in Table 4. Odds of diabetes diagnosis in CP increased with increasing age of pancreatitis symptom onset, duration of disease, BMI category of overweight or obese, positive family history in first degree relative, pancreatic calcifications, prior pancreas surgery, and exocrine insufficiency. The most notable risk factors for diabetes included obesity by BMI ≥ 30 kg m−2 vs. normal BMI (OR 2.8, 95% CI 1.9, 4.2), exocrine insufficiency (OR 2.4, 95% CI 1.8, 3.2).
Table 4.
OR of patient and disease factors for presence of diabetes derived from multivariable logistic regression modelling in the 1076 patients with complete data
| Variable | OR | 95% CI | P-value |
|---|---|---|---|
| Age at onset of pancreatitis symptoms (years) | 1.02 | (1.014, 1.035) | <0.0001 |
| Duration of pancreatitis | 1.05 | (1.026, 1.069) | <0.0001 |
| Underweight BMI (vs. normal) | 0.77 | (0.466, 1.285) | 0.32 |
| Overweight BMI (vs. normal) | 1.62 | (1.160, 2.268) | 0.005 |
| Obese BMI (vs. normal) | 2.83 | (1.895, 4.215) | <0.0001 |
| Male gender | 1.32 | (0.983, 1.777) | 0.07 |
| Heavy or very heavy alcohol use (vs. no use) | 1.00 | (0.670, 1.492) | 0.99 |
| Light to moderate alcohol use (vs. no use) | 0.60 | (0.396, 0.907) | 0.02 |
| Pancreatic calcifications | 1.58 | (1.182, 2.116) | 0.002 |
| Exocrine insufficiency | 2.38 | (1.785, 3.162) | <0.0001 |
| Pancreas surgery history | 1.75 | (1.244, 2.469) | 0.001 |
| Family history of diabetes | 1.48 | (1.113, 1.974) | 0.007 |
BMI, body mass index; CI, confidence interval; OR, odds ratio.
BMI categories are defined based on BMI at time of study enrollment.
An additional interaction term for BMI category and exocrine insufficiency added to the final model, to test whether the effect of BMI on diabetes differed based on the presence of exocrine insufficiency. This interaction term was insignificant (P =0.576). In other words, overweight and obese BMI category increased the risk of diabetes and exocrine insufficiency increased the risk of diabetes, but these two risk factors were independent and additive.
The association of BMI and family history was strongest in the subset of patients who had diabetes diagnosed before the diagnosis of CP. In this group of patients, obesity by BMI vs. normal BMI conveyed an OR of 3.3 (95% CI 1.59, 6.97) and positive family history of diabetes with an OR of 1.69 (95% CI 0.99, 2.88) for the presence of diabetes. In contrast, in the subset of patients diagnosed with diabetes after CP diagnosis, these associations lost both strength of association and statistical significance: OR 1.41 (0.57, 3.49) for obesity vs. normal BMI, and OR 0.85 (0.46, 1.59) for family history. The disease factors calcifications and exocrine insufficiency are strongly associated with increased odds of diabetes regardless of whether diabetes was diagnosed before or after CP. For pancreatic calcifications, the OR was 2.54 (1.39, 4.64) when diabetes was diagnosed before CP and 2.19 (1.15, 4.15) when diabetes was diagnosed after CP; for exocrine insufficiency, the OR was 2.50 (1.46, 4.26) when diabetes was diagnosed before CP and 3.06 (1.71, 5.45) when diabetes was diagnosed after CP.
Table 5 uses information from the logistic regression model to illustrate the incremental role of the traditional risk factors above and beyond the diseases-related attributes on the probability of diabetes in a representative 50-year-old male with a 5-year duration of disease—the probability of diabetes in a self-reported heavy or very-heavy male drinker during the maximum drinking period in life with normal weight, no family history, no calcifications, exocrine insufficiency, or pancreatic surgery is 15%, which increases to 42% in the presence of being obese and with a family history, to 74% in the presence of calcifications and exocrine insufficiency, and 83% if the patient also had pancreatic surgery. For comparison, the risk for DM in an average 50-year-old male with the US population is about 12% (5).
Table 5.
Illustrative example of risk for DM in a 50-year-old male with CP of 5 years duration and self-reported heavy/very-heavy drinking with various combinations of risk factors
| Attribute* | |||||
|---|---|---|---|---|---|
| Calcifications | Exocrine insufficiency | Pancreatic surgery | BMI category | Family history of diabetes (first degree) | Predicted probability of diabetes (%) (95% CI) |
| No | No | No | Underweight | Yes | 17 (10–26) |
| No | Yes | No | Underweight | Yes | 32 (21–46) |
| No | No | No | Normal | No | 15 (11–21) |
| No | No | Yes | Normal | No | 34 (16–33) |
| Yes | Yes | No | Normal | No | 40 (32–48) |
| Yes | Yes | Yes | Normal | No | 54 (43–64) |
| No | No | No | Normal | Yes | 31 (15–28) |
| No | No | No | Overweight | Yes | 30 (22–39) |
| No | No | No | Obese | Yes | 43 (32–54) |
| No | No | Yes | Obese | Yes | 56 (43–69) |
| Yes | Yes | No | Obese | Yes | 74 (63–82) |
| Yes | Yes | Yes | Obese | Yes | 83 (73–90) |
CI, confidence interval; CP, chronic pancreatitis; DM, diabetes mellitus.
For a given set of attributes, the probability in a lifetime abstainer is almost similar to, and for a light–moderate drinker ~5–10% lower than heavy/very-heavy drinker. Probability of diabetes will be unique to an individual based on the combination of different attributes.
As there were 37 cases where patients self-reported DM, while the physician did not report DM for the same individual, we performed a sensitivity analysis by repeating this multilinear logistic regression modeling, excluding these 37 participants. The results without these 37 individuals were essentially unchanged.
DISCUSSION
DM is a long-recognized complication of CP, resulting from progressive pancreatic fibrosis with reduced β-cell mass and impaired insulin secretion. In this large North American cohort of patients with CP, fully one-third of participants with CP had DM. This was progressive with age of enrollment, with ~18% of those participants <40 years of age affected, increasing to 35–>40% affected above the age of 40 years. Yet, at any age, this risk was high when compared with the general population risk in the United States (5). Key pancreatitis disease factors such as morphologically more severe disease, exocrine insufficiency, and prior pancreatic surgery increased the odds for diabetes, but so did traditional risk factors for type 2 diabetes including obesity and family history of diabetes.
Similar to our findings, pancreatic calcifications, pancreatic surgeries, and pancreatic exocrine insufficiency have all been previously associated with increased risk for development of DM (7, 14). When studied with detailed metabolic and digestive phenotyping, in patients with advanced CP, lower C-peptide levels stimulated by oral glucose and intravenous secretagogues correlate with exocrine insufficiency defined by lower measured amylase and lipase output. This, as well as clinical observations of increased prevalence of DM with exocrine insufficient CP, support the postulated mechanism of progressive fibrosis of the pancreas damaging both the acinar and islet components of the pancreas (14). In our population, exocrine insufficiency was a risk factor for diabetes in all categories of BMI—exocrine insufficiency was diagnosed in a significant proportion of patients in all categories of BMI (under-weight, normal weight, overweight, and obese) and increased the risk for diabetes within each subgroup. With regards to other disease morphology and treatments, Malka et al. (7) previously reported a two-to three fold increased risk of DM with pancreatic calcifications and when distal pancreatectomy was performed in patients with primarily alcoholic-mediated CP. Importantly, our findings validate these observations in a larger North American cohort with diverse causes of CP.
The most common form of diabetes in the United States is type 2 DM—accounting for the majority of the 9.3% of adults who have DM in the United States (5). Type 2 DM is characterized by insulin resistance with a relative beta cell failure in that the pancreatic β-cells are unable to increase insulin secretion suffi ciently to overcome insulin resistance (15). Type 2 DM is more common in certain racial minority populations, obese individuals, and those with a family history of diabetes. In contrast to previous studies where traditional T2DM risk factors were not a focus, we identified a substantial increase in the odds of diabetes in our CP patients when obesity (nearly threefold odds of DM), over-weight status (1.6-fold increase), or positive family history of DM (1.5-fold increased odds of DM) were present. In the multivariate model, obesity was actually a stronger risk factors for DM than the pancreatic disease features of calcifications, prior surgery, or exocrine insufficiency. This association was particularly driven by the participants who were diagnosed with diabetes before CP diagnosis, suggesting a stronger tendency towards a ‘type 2 phenotype’ when diabetes is diagnosed early; however, calcifications and exocrine insufficiency were still strongly associated with DM in these patients who had diabetes prior to the diagnosis of CP, suggesting exocrine parenchymal disease as an additive risk factor. Although Black race was also more frequent in DM, this did not emerge as a significant variable in the multivariable analysis. We postulate that risk factors for type 2 DM could increase or hasten the presentation of type 3c DM (pancreatogenous diabetes), due to a ‘double-hit’ of impaired pancreatic beta cell mass from CP plus insulin resistance or genetic impairments in β-cell function. This ‘double hit’ process may be particularly important in those diagnosed with diabetes earlier, whereas later diabetes may be largely driven by the pancreas parenchymal injury from the CP process itself.
As might be anticipated, the patients who were diabetic were, on average, older and with a longer duration of disease. This is consistent with previous literature, suggesting that prevalence of diabetes increases with increased duration of disease (16). Of note, the presumed etiology of disease was not an important factor in predicting risk for DM in our patient cohort. Hyperlipidemia was reported more frequently as a primary suspected cause for pancreatitis in those with DM, as might be expected due to the relationship between hyperlipidemia and DM (17, 18), but this was a rare etiology for CP. The more common etiologies of alcohol use, idiopathic CP, and genetic disease were similarly distributed among CP patients with and without DM.
Surprisingly, in multivariate regression modeling, we found a lower odds for diabetes in participants identified as low to moderate alcohol drinkers compared to alcohol abstainers (OR for diabetes of 0.60). The reason for this association is unclear. In type 2 diabetes research, light consumption of red wine (one glass per day) has been associated with a significant reduction in fasting glucose compared with placebo (19), and in healthy controls a short-term infusion of alcohol to a blood alcohol level of 0.08 suppresses gluconeogenesis and endogenous glucose production during conditions of hyperglycemia (20), suggesting theoretical potential for light alcohol use to lower blood glucose. Although this is speculative, it is consistent with literature in type 2 diabetes, suggesting a potential protective effect of light to moderate alcohol consump tion against diagnosis of type 2 diabetes (21). Conversely, this association may be driven by a yet-unidentified confounder associated with light alcohol consumption that reduces diabetes risk.
A novel aspect to the NAPS2 cohort is that we collected quality-of-life scores on patients by the short-form 12 as part of the patient questionnaires (22). In univariable analysis, on the Short Form-12, the mean physical component summary score was slightly lower in those with DM, suggesting that the combination of DM+CP adds greater physical disease burden than CP alone. However, in a recent analysis of factors determining quality of life in CP patients (paper under review), DM was not a significant factor in a multivariable model. Moreover, the patients with CP and DM were not on disability coverage any more oft en than CP alone, perhaps reflecting that the rate of disability in CP was already overall high (about one in every four participants).
Although we postulate that obesity and genetic risk, reflected by a family history of DM, are risk factors for developing type 3c DM as well, a subset of our population may simply have classic type 2 DM since type 2 is present in about 9% of the general US population. Within the limitations of this epidemiologic cohort, we do not have any tests to distinguish type 2 DM from type 3c DM from ‘double/overlapping’ type 2/ type 3c DM. We also did not assess whether beta cell autoimmunity (type 1 diabetes) was a contributor to diabetes development in this cohort; however, interestingly, 1 in every 106 study participants was diagnosed with DM at age <21 years, fourfold higher than the expected rate of childhood onset diabetes of 1 in every 418 children in the United States in 2009 (23), even though most were not yet diagnosed with CP at time of childhood onset DM. Future studies—including those emerging from the Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) research consortium—will be necessary to better define diagnostic protocols to accurately classify type 3c vs. type 2 DM, and to explore whether markers of β-cell autoimmunity are more prevalent in those with CP, as has been reported recently for cystic fibrosis-related diabetes (24), and whether markers such as pancreatic polypeptide can adequately distinguish true type 3c vs. classic type 2 DM in those with CP.
Our cohort is also limited by the cross-sectional nature of the analyses to identify associations with DM—further large-scale longitudinal studies will be needed to confirm these risk factors, determine more specifically the timing of onset of disease, and relation to CP disease and duration. As delayed diagnoses are possible for both CP and diabetes, the dates of diagnosis that were provided by the physician may not accurately reflect the time course of actual CP and diabetes onset, thus confounding our associations. The prevalence of DM in our cohort may be an underestimation as formal testing for diabetes was not a requirement as part of the NAPS2 study enrollment. In addition, recall bias may occur with assessment of family history of diabetes or hyperlipidemia as a cause of CP in those who are obese or already diabetic (i.e, have health complications that may be associated with these features). Likewise, pancreatic exocrine insufficiency (a risk factor for higher odds DM) was defined by physician report and thus it is possible that exocrine insufficiency was over or underdiagnosed in the cohort.
Overall, our results support the previously cited high rate of DM in CP, much higher than the general population. Previously suspected risk factors for DM such as exocrine insufficiency, calcifications, and pancreas surgery were observed in this large diverse cohort. However, we identified traditional ‘type 2 DM’ risk factors of obesity and family history as equally contributing to the odds of developing DM in the context of CP, especially for those diagnosed with diabetes early.
Supplementary Material
WHAT IS CURRENT KNOWLEDGE?
Patients with chronic pancreatitis (CP) are at high risk for developing diabetes mellitus (DM).
Pancreatic exocrine insufficiency, pancreatic surgery, and pancreatic calcifications have been associated with higher risk for DM, but such studies are often in select populations (alcoholic disease, or hereditary disease).
WHAT IS NEW HERE.
Patients (1,171) with CP with diverse causes for CP, seen at 26 pancreatic care centers in the United States were studied for prevalence of DM and risk factors for DM.
Pancreatic exocrine insufficiency, pancreatic calcifications, and pancreatic surgery were risk factors for DM.
This is the first study to identify traditional ‘type 2’ DM risk factors of obesity and positive family history of DM associated with risk for DM in CP. However, the impact of obesity and family history is strongest when diabetes precedes the diagnosis of CP.
A ‘double hit’ of pancreatic damage from CP and traditional type 2 DM risk factors may increase the risk for DM in the setting of CP.
Acknowledgments
Financial support:
The study was supported by R01DK061451 (D.C.W.), R01 DK077906 (D.Y.), UO1 DK108306 (D.C.W. and D.Y.), UO1 DK 108327 (D.C), UO1 DK108320 (C.E.F.), and UL1 RR024153 and UL1TR000005 (P.I.—Steven E. Reis, MD).
Footnotes
Guarantor of the article: Dhiraj Yadav, MD, MPH.
CONFLICT OF INTEREST
Potential competing interest: Whitcomb is an inventor of intellectual property that is licensed to Ambry Genetics, which has been evaluated in this study. He also has an ownership interest in Ambry Genetics. All other authors have no conflicts of interest related to the manuscript.
REFERENCES
- 1.Braganza JM, Lee SH, McCloy RF et al. Chronic pancreatitis. Lancet 2011; 377: 1184–97. [DOI] [PubMed] [Google Scholar]
- 2.Schrader H, Menge BA, Zeidler C et al. Determinants of glucose control in patients with chronic pancreatitis. Diabetologia 2010; 53:1062–9. [DOI] [PubMed] [Google Scholar]
- 3.Meier JJ, Breuer TG, Bonadonna RC et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia 2012; 55: 1346–54. [DOI] [PubMed] [Google Scholar]
- 4.Domschke S, Stock KP, Pichl J et al. Beta-cell reserve capacity in chronic pancreatitis. Hepatogastroenterology 1985; 32:27–30. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National Diabetes Statistics 2014. Accessed at https://www.cdc.gov/diabetes/data/national.html.
- 6.Hardt PD, Bellin MD, Andersen DK et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016; 1:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malka D, Hammel P, Sauvanet A et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology 2000; 119:1324–32. [DOI] [PubMed] [Google Scholar]
- 8.Howes N, Lerch MM, Greenhalf W et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol 2004; 2:252–61. [DOI] [PubMed] [Google Scholar]
- 9.Rebours V, Boutron-Ruault MC, Schnee M et al. The natural history of hereditary pancreatitis: a national series. Gut 2009;58:97–103. [DOI] [PubMed] [Google Scholar]
- 10.Wakasugi H, Funakoshi A, Iguchi H. Clinical assessment of pancreatic diabetes caused by chronic pancreatitis. J Gastroenterol 1998; 33:254–9. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CM, Sandhu BS, Singh V et al. Racial differences in the clinical profile, causes, and outcome of chronic pancreatitis. Am J Gastroenterol 2016; 111:1488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox CM, Yadav D, Ye T et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015; 13:552–60; quiz e28–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitcomb DC, Yadav D, Adam S et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nyboe Andersen B, Krarup T, Th orsgaard Pedersen NT et al. B cell function in patients with chronic pancreatitis and its relation to exocrine pancreatic function. Diabetologia 1982; 23:86–9. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014; 383:1068–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan J, Xin L, Wang D et al. Risk factors for diabetes mellitus in chronic pancreatitis: a cohort of 2011 patients. Medicine 2016; 95:e3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haffner SM. Diabetes, hyperlipidemia, and coronary artery disease. Am J Cardiol 1999; 83:17f–21f. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg RB. Hyperlipidemia and cardiovascular risk factors in patients with type 2 diabetes. Am J Manag Care 2000; 6 (13 Suppl):S682–91; discussion S92–6. [PubMed] [Google Scholar]
- 19.Shai I, Wainstein J, Harman-Boehm I et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multi-center, rando mized, clinical intervention trial. Diabetes Care 2007; 30: 3011–6. [DOI] [PubMed] [Google Scholar]
- 20.Kehlenbrink S, Tonelli J, Koppaka S et al. Inhibiting gluconeogenesis prevents fatty acid-induced increases in endogenous glucose production. Am J Physiol Endocrinol Metab 2009; 297:E165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koppes LL, Dekker JM, Hendriks HF et al. Moderate alcohol consumption lowers the risk of type 2 diabetes: a meta-analysis of prospective observational studies. Diabetes Care 2005;28:719–25. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Sherbourne CD. Th e MOS 36-item short-form health survey (SF-36). Med Care 1992; 30:473–83. [PubMed] [Google Scholar]
- 23.Dabelea D, Mayer-Davis EJ, Saydah S et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014; 311:1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konrad K, Kapellen T, Lilienthal E et al. Does beta-cell autoimmunity play a role in cystic fibrosis-related diabetes? Analysis based on the German/Austrian Diabetes Patienten Verlaufsdokumentation Registry. Diabetes Care 2016;39:1338–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


