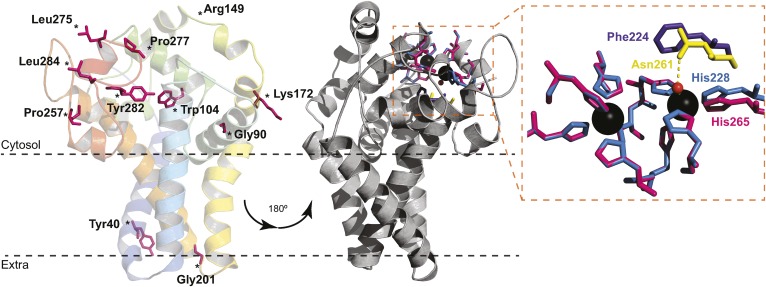
Fig. 5.
Structure homology model of B. cereus DesA. The predicted model of acyl-lipid desaturase DesA from B. cereus was performed with the program Phyre2, using the crystal structure of the human SCD1 as a template (Protein Data Bank ID code 4ZYO). The 72% of residues were modeled (18–284 AA) with 99.9% confidence. The model is represented as cartoon and colored in rainbow from N terminus (blue) to C terminus (red). The conserved mutagenized residues are shown as pink sticks. The gray representation is rotated 180°, and the residues involved in metal coordination are represented as sticks (left). Close-up view of the catalytic site pocket (right) shows the nine His residues of SCD1 (pink) and DesA (blue). Phe224 of DesA (purple) cannot bind a water molecule as Asn261 of SCD1 (yellow) to complete the coordination sphere.