Abstract
BACKGROUND AND OBJECTIVE
The soluble Epoxide Hydrolase (sEH) is an enzyme in the arachidonate cascade which converts epoxy fatty acids (EpFA) such as epoxyeicosatrienoic acids (EETs) produced by cytochrome P450 enzymes to dihydroxy-eicosatrienoic acids (DHETs). In last years with the development of inhibitors to sEH was possible to increase of EETs and other EpFA levels in vivo models. Recently, studies have shown that EETs play a key role in blocking inflammation in a bone resorption process, but this mechanism is not clear. In the current study we used the sEH inhibitor (TPPU) to investigate the immunomodulatory effects in a mouse periodontitis model.
MATERIAL AND METHODS
Mice were infected on days 0, 2, and 4 with Aggregatibacter actinomycetemcomitans and divided into groups (n = 6) that were treated daily orally during 15 days with 1 mg/kg TPPU. The animals were sacrificed, and the jaws were analyzed for bone resorption by morphometry. Immunoinflammatory markers in the gingival tissue were analyzed by microarray PCR or Western blotting.
RESULTS
Infected animals treated with TPPU showed lower bone resorption than infected animals without treatment. Interestingly, infected animals showed increased expression of sEH however; animals treated with TPPU had a reduction of the protein expression sEH. Besides, several proinflammatory cytokines and molecular markers were downregulated in the gingival tissue in the group treated with 1 mg/kg TPPU.
CONCLUSIONS
The sEH inhibitor (TPPU), showed immunomodulatory effects, decreasing bone resorption and inflammatory responses in a bone resorption mouse model.
Keywords: bone, periodontitis, inflammation, epoxyeicosatrienoic acid, soluble epoxide hydrolase, TPPU
Introduction
The human oral cavity harbors a substantial and continuously evolving load of microbial species. Many infectious diseases, including periodontal diseases appear to be infections mediated by the overgrowth of commensal organisms, rather than by the acquisition of an exogenous pathogen. As microorganisms evolve more rapidly than their hosts do, immune mechanisms that determine the ecological balance of commensal organisms also need to change to preserve homeostasis [1]. The immune system involvement will depend on the severity and progression of periodontal disease and may have the participation of both the innate and the adaptive immune response. The knowledge of how immune mechanisms and inflammatory responses are regulated is important for understanding the pathogenesis of complex diseases, such as periodontitis; in addition, it is important to continue considering mechanisms that regulate inflammatory responses because they may open up novel therapeutic targets.
Epoxyeicosatrienoic acids (EETs) are biologically active metabolites of arachidonic acid, generated by the activity of cytochrome p450 (CYP) enzymes. Once formed, EETs are unstable, being rapidly converted into less active or inactive dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH) [2]. Still, this conversion may be prevented by inhibitors of sEH (sEHI), so EETs can be stabilized, prolonging their biological effects [3]. Studies on these effects emphasize ETTs role in vasodilatation of systemic vessels and control of blood pressure, grounding their promise in the treatment of vascular diseases, such as atherosclerosis and hypertension [2,3]. Emerging additional benefits have been described for ETTs, which are currently recognized as potent anti-inflammatory agents [2,4]. They can inhibit cytokine-induced inflammation signaling mediated by NF-ⱪB, reducing the expression of proinflammatory mediators and cell adhesion molecules [5]. Systemic overexpression of human CYP2J2, a CYP epoxygenase isoform that produces EETs, led to a significant reduction of the TNFα-induced high plasma levels of adhesion molecules and inflammatory cytokines in rats, suggesting that EETs and other EpFA act as anti-inflammatory mediators [6]. In addition, sEH pharmacological inhibitors, such as 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea - TPPU, have been the focus of deep investigation, since by reducing the metabolism of EETs, these inhibitors were demonstrated to alleviate inflammatory and fibrotic diseases in animal models [7–10]. Therefore, sEH may be an important target for the modulation of periodontal disease and bone resorption.
In this study, using an animal model of periodontitis, we explored the molecular mechanisms by which TPPU may modulate the immunological response to avoid the disease-related bone loss.
Material and Methods
Animals
Male mice were maintained under standard conditions, at 12-h light–dark cycle with food and water ad libitum. Six mice per group weighing 20-25 g were age-matched. All procedures were in agreement with standards for the care of laboratory animals as outlined in the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Resource Services of the University.
Periodontitis model and treatments
Aggregatibacter actinomycetemcomitans (JP2) were cultured under anaerobic conditions to ensure viability and virulence. Animals orally received 1×109 CFU/ml of A. actinomycetemcomitans diluted in 100 μl PBS containing 2% carboxymethylcellulose. Oral colonization of A. actinomycetemcomitans was performed before at day 0 and at the end of the experiment, as previously described [11]. The solution was placed into the oral cavity, and the procedure was repeated at 2 and 4 days after the first inoculation. Treatment with 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea (TPPU) which was dissolved in PEG400, was initiated after the last inoculation of bacteria by oral gavage. The dose of TPPU used was 1mg/kg/day during 15 days as previously tested [10]. The negative control group consisted of uninfected mice (sham-infected) and the positive group was infected and received the vehicle only (PEG400). The day after the treatment period, animals were sacrificed by cardiac puncture after anesthesia with xylazine/ketamine. The whole buccal and palatal periodontal tissues of the upper and lower molars were collected, weighed, and the portions of gingival tissue were separated half portion for protein extraction and the other half portion for mRNA extraction. The amount of gingival tissue for protein extraction were triturated and homogenized in 300 μL of the appropriate buffer containing protease inhibitors (Sigma-Aldrich), followed by centrifugation for 10 minutes at 10,000 g. The supernatant was rapidly frozen, and stored at -70°C for further gene expression analyses or western blotting. Animals were continuously weighed.
Quantification of alveolar bone loss
Evaluation of alveolar bone loss was performed as described previously [11]. The jaws were removed, and defleshed, then immersed overnight in 3% hydrogen peroxide, washed in PBS and followed by 1% methylene blue staining. The horizontal bone loss was assessed morphometrically by measuring the distance between the cement–enamel junction and the alveolar bone crest of the first and second molars. Measurements at 14 buccal sites per mouse were made under a microscope, and bone measurements were analyzed using the Image J software. Random and blinded measurements were taken by the same calibrated person achieving intra-examiner reproducibility >90%.
Western Blotting
Tissues were lysed and clarified by centrifugation and protein concentrations were determined using the bicinchoninic acid protein assay kit (ThermoFisher Scientific, Waltham MA). Equal amounts of protein (20 μg) from the gingival tissue were resolved by SDS-PAGE and transferred to PVDF membranes and then incubated with rabbit anti-mouse soluble epoxide hydrolase (sEH) antibody (1:1000; Dr Hammock, University of California, Davis) or α-Tubulin (1:1000; Santa Cruz Biotechnology; Santa Cruz, CA). After incubation with the goat anti-rabbit secondary antibodies, proteins were visualized using Luminata™ Fort Western HRP substrate (Millipore). Pixel intensities of immunoreactive bands were quantitated using FluorChem Q Imaging software (Alpha Innotech). Data were presented as total protein expression normalized to α-Tubulin [12].
PCR Array
The RNeasy Micro Kit (Qiagen) was used for total RNA extraction, and 300 ng were retrotranscribed using the RT2 First Strand Kit (Qiagen) following the manufacturer’s instructions. For the study of gingival tissue gene expression, the 96-well Qiagen PCR Array Mouse Innate & Adaptive Immune Responses was used in combination with the RT2 SYBR Green qPCR Mastermix (Qiagen), using 10 ng of cDNA per well. Results for each assay were normalized to the average of all five housekeeping genes. Gene expression changes were reported if a 3.0-fold threshold was detected.
Statistical analysis
Results were expressed as the mean ± standard deviation. Data were by the Shapiro-Wilk test and found to be normally distributed. One-way analysis of variance (ANOVA) with post hoc Tukey test was applied, with a significance level of 0.05.
Results
The effect of oral administration of TPPU on A.a-induced periodontal bone resorption animals was assessed by quantifying in all mice the distance between CEJ (cement-enamel junction) and ABC (alveolar bone crest) during the experimental period. Animals sham-infected showed no bone resorption during the experimental period (Figure 1). On the other hand, animals orally infected with A. actinomycetemcomitans showed high bone resorption which was completely abrogated in the group orally treated with TPPU (Figure 1). Animals were constantly weighed during the experimental period, and there was no statistical variation among the groups (Figure 2, p > 0.05).
Figure 1.
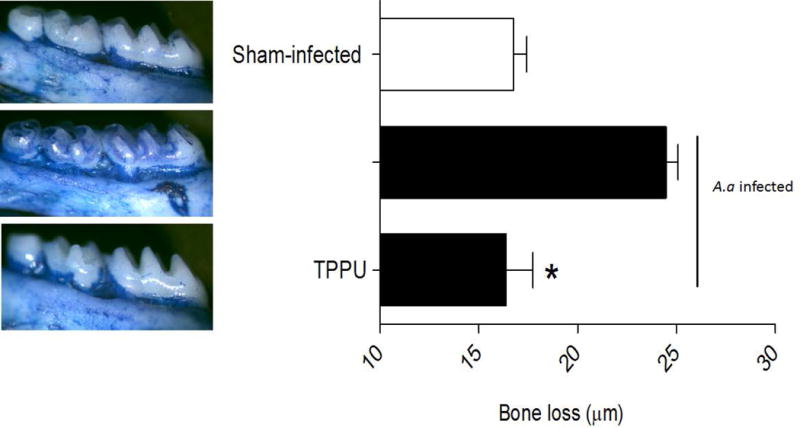
TPPU decreases bone loss by inhibition of sEH. Distance (μm) between the cement-enamel junction and the alveolar bone crest for all experimental groups were quantified. The results are expressed as mean ± SD. *p <0.05 among the infected-animals; non-infected and infected group treated with 1mg/kg TPPU.
Figure 2.
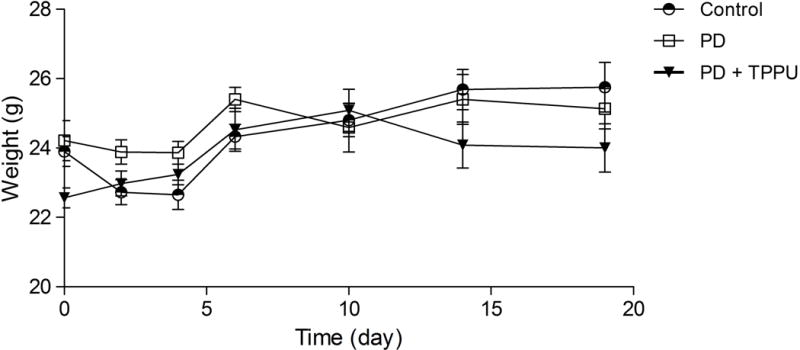
Inhibition of sEH and periodontal disease did not change animal weight. Animal weights were monitored along the experimental period. The results are expressed as mean ± SD.
Western blot analysis from gingival tissue demonstrated that animals orally infected with A. actinomycetemcomitans had an increase of the protein expression of the epoxide hydrolase which was abrogated in the infected animals treated with sEH inhibitor (p < 0.05) as demonstrated in Figure 3A. The data from band density is expressed as an arbitrary unit (Figure 3B).
Figure 3.
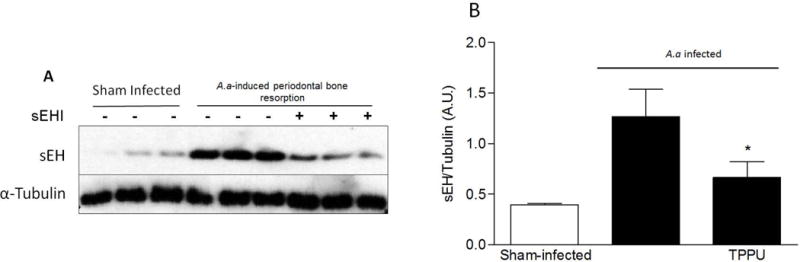
Protein expression of EH in gingival tissues. Density of the EH bands were normalized to α-tubulin. Protein band intensity is represented as arbitrary units (mean ± SD). *p <0.05 among the infected-animals and non-infected and infected group treated with 1mg/kg TPPU.
A PCR Array analysis was performed to evaluate the profile of the expression of 84 genes involved in the host response to bacterial infection. Applying a 3-fold change cut-off value for the differential expression, we identified 6 genes up-regulated in the animals infected and treated with TPPU in comparison to infected animals: IL-13 and IL-23, SAP, IFN-β, Mbl-2, and MPO. On the other hand, 18 genes were down-regulated in the group treated with TPPU in comparison to the infected but not treated: CD8, CD40Ig, CCR6, CD4, CCR8, Lyz2, TLR1, STAT4, IFNγ, TLR9, CCR4, IL-5, IFNα2, CD40, CXCR3, GATA3, RAG-1, Mx1. Figure 4 summarizes the genes whose expression was affected by TPPU. It is worth highlighting that the FOXP3 expression was down regulated at borderline to be considering statistical significant (~ 2.8 fold).
Figure 4.
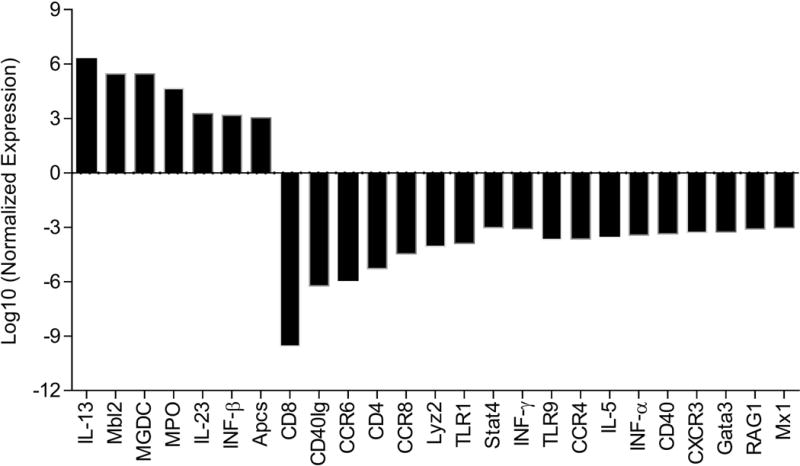
Effects of treatment TPPU on the expression of a panel of inflammatory genes on gingival tissue. Values are expressed as fold change in gene expression between A.a-induced periodontal bone resorption animals and those treated with TPPU. Negative values represent gene down-regulation, and positive values represent gene up-regulation. A 3-fold difference cut-off point was set.
Discussion
The participation of the immune system in the face of the aggressive growth and tissue damage that the microorganisms can cause is an important factor since many of the immunological factors involved in response to the bacteria can cause a change in homeostasis and affect the alveolar bone [13].
Studies have shown that EETs play a key role in the anti-inflammatory process and would have a beneficial effect to prevent bone loss caused by PD [10,14,15]. The EETs are rapidly metabolized by the enzyme soluble epoxide hydrolase (sEH), and thus the EETs have a short half-life, making the natural EETs difficult to use pharmacologically. Because of that, it was employed the development of TPPU, to increase the half-life of EETs and thus enhance their biological effects [16]. As demonstrated, the A.a-induced periodontal disease showed an increase in the sEH expression which was abrogated when the animals were treated with TPPU. This is the first report demonstrating the expression of the sEH in the periodontal tissues and thus the importance of the EET/sEH to the homeostasis of the periodontium.
The bacterial biofilm initiates PD, but the destruction of the tissue in the periodontal lesions may occur because of the destructive processes mediated by the host’s immune response [17]. The mannan binding lectin (MBL) was upregulated in animals that were treated with TPPU. This molecule stimulates the classical complement pathway as an opsonin and plays a role in the defense against invading microorganisms in periodontitis. Thus, MBL can recognize carbohydrate structures, on a variety of microorganisms surface [18]. In according to our findings in the gingival tissue, the MBL plasma levels have been reported to increase during infections and inflammatory processes [19]. MBL plasma concentrations were not significantly different in moderate and severe periodontitis compared to controls, and MBL deficiency was not related to susceptibility for periodontitis [20]. Once our disease model was provoked by inoculating A. actinomycetemcomitans this may explain the dramatically increasing MBL expression.
Significant attention has been given to pattern recognition receptors similar to the Toll Receptors (TLRs) [21]. TLR 1 and 9 were significantly down regulated in the TPPU-treated mice. TLRs in PD play a key role in triggering the inflammatory response since they can mediate the release of inflammatory cytokines [17]. Recently, TLR2 was shown to form heterodimers with TLR1 and TLR6 that recognize triacylated lipopeptides from Gram-negative bacteria [22]. Interesting, TLR2/1 ligand induce osteoclastic bone resorption in mouse culture cells [23].
Human type I interferons (IFNs) are made and released by host cells in response to the presence of several pathogens. In inflammation IFNs possess both pro- and anti-inflammatory functions depending on the context of the particular pathology [24]. Recently, an inhibitory role for type I IFNs in blocking IL-1β production has been reported [25]. In this study, the IFN-β1 proteins are up-regulated, suggesting the importance of TPPU in controlling the inflammatory process in the gingival tissue.
T helper 1 cells secrete IL-2 and IFN-γ [26] and there is strong evidence that the release of IFN-γ, resulting in increasing loss of alveolar bone during periodontal infections [27]. Besides, high levels of IFN-γ in patients with periodontitis, is related to the severity of the disease [28]. Thus, the decreasing expression of IFN-γ by TPPU may explain the decrease of bone resorption. In addition, the TPPU also reduced the expression of receptors CD4 and CD8 and consequently lymphocytes.
The chemokine receptor (CCR), is expressed in a pathological situation and is responsible for the development and function of the immune system during the inflammatory process [29,30]. The administration of TPPU decrease the CCR6, CCR4 and CCR8 expression. Studies show that CCR6 is a specific marker for Th17 and regulatory T cells [31]. Furthermore, we demonstrated that treatment with TPPU also modulated the CXCR3 receptor. This receptor is responsible to guide cells out of the lymphoid compartment towards the sites of inflammation mainly CD8+ T cells [32].
The CCR4 and CCR8 are expressed predominantly in Th2 cells and Treg. These two chemokines provide guidance to the T cells that express CCR4 so that they can reach the inflamed mucosa. The CCL17 promotes vascular recognition and emigration of T cells that express CCR4 in endothelial surface, while the CCL22 migration leader these same cells in mucosal tissue [33]. The CCR8 participates in induction and amplification of the inflammatory responses to pathogens or allergens recruiting effector memory T cells. Moreover, it participates in the recruitment of regulatory T cells, contributing the balance between effector and regulatory cells, and thus the outcome of the inflammatory response [34]. Natural Treg cells are CD4- and CD25-FOXP3 expressing T-cells that specifically regulate the activation, proliferation and effector functions of activated conventional T-cells [35] and T-regulatory cells found in periodontal disease sites [36]. Our results demonstrated that animals treated with TPPU statistically decreased the expression of CCR4 and CCR8 and with borderline significance FOXP3. It is important to note that the Treg influx was decreased in the gingiva of the treated animals. Previous results corroborate that immunomodulatory drugs such as 15d-PGJ2 [37] drove a shift to a rapid resolution of inflammation, which causes the natural decrease of Treg cells at the inflammatory site.
The interleukins IL-4, IL-5, and IL-13 are expressed by the gene GATA-3 which is the only recognized by transcription factor expression in Th2 cells [38]. The subtype RAG1 is a gene involved in genetic recombination, in a process that leads to the organization of antigen receptors genes. This gene along with the GATA-3 were suppressed by TPPU and demonstrates its importance of this pathway when you take into account the adaptive immune system is characterized by ability in forming antigen-specific receptors. In the absence of GATA-3, the differentiation of CD4+ is deficient before the CD4/CD8 interaction, because the thymocytes with deficiency of GATA-3 and restricted to the MHC-II are redirected to the lineage of CD8+ [39,40].
The CD40 and CD40L were also down regulated in the animals treated with TPPU. The CD40L is expressed on the surface of CD4+ cells and binds to CD40 on B cells to provide the B-cell activation and differentiation and the production of antibodies against pathogens [41,42]. The activated T and B lymphocytes in gingival tissue of patients with periodontitis express RANKL [43,44], as well as SOFAT [45] both major contributors to the activation of osteoclast cells. Besides, CD40 binding stimulates the production of cytokines by B cells, including IL2, IL6, IL10 and TNF-α, some of these essential in the development of periodontal disease [46].
In our study it was demonstrated that a classical standard Th2 cytokine, IL-5 was also decreased in the group of animals treated with TPPU. This cytokine along with IL-6 and TNF-α can promote survival of plasma cells in the presence of inflammation [47]. The effects of IL-5 include the maintenance of survival and functions of B cells and eosinophils, and a lack of functional genes or receptors to IL-5, induce deficiencies in the development of these cells [48].
Interleukin-13, which is up-regulated in the TPPU-treated mice, is another potent modulator of human monocyte/macrophage and B-cell function. Monocyte/macrophage MHC class II and several integrin molecules are up-regulated by IL-13 [49], however, the production of IL-1α, IL-1β, IL-6, IL-8 and TNF-α are inhibited by IL-13, suggesting an anti-inflammatory role [49].
The plasma protein serum amyloid P (SAP) reduces neutrophil adhesion, inhibits the differentiation of monocytes into fibroblast-like cells, and promotes phagocytosis of cell debris by macrophages. Together, these effects of SAP reduce important aspects of inflammation and fibrosis [50] which help to explain the decreased bone resorption. sEH inhibitors have been shown multiple studies to reduce inflammation and fibrosis [2,4,7,9,10].
In addition was also observed that decrease of transcription signal transducer and activator 4 (STAT4), a transcription factor for interleukins IL-12, IL-23 and IFN1, leading to differentiation of Th1 cells and Th17, as well as to activate monocytes and lead to the production of IFN-γ. By leading to the production of IFN-γ and IL-17, this transcription factor directs T cells to the Th1 cell lines. STAT4 in other cellular subtypes can also contribute to the progression of diseases due to the increase of inflammatory cytokines, preventing apoptosis, presentation of antigens or antibody production [51]. The immunomodulation of this STAT is an important clue of the effect of TPPU.
Other aspect to be considered in our study were the decreased expression of the Lyz2 (Lysozyme M) and MX1 genes in the animals treated with TPPU. The unbalanced patterns of Lyz2 lead to increased bacterial load and consequently to persistent inflammation. Although not very clear in the literature, the MX1 gene is related to inflammatory disease mediated by T cells [52]
Taken together, our results demonstrate that the inhibition of the soluble epoxide hydrolase enzyme by TPPU may contribute to control the inflammatory process by modulating cell activation and influx as well as the production of specific cytokines, suggesting that inhibition of sEH could be a therapeutic approach for periodontal disease treatment and bone loss and other inflammatory conditions.
Acknowledgments
Financial support
Brazilian funding agencies São Paulo Research Foundation (FAPESP) and National Council for Scientific and Technological Development (CNPq); MHN was supported by grant [2015/23556-0](FAPESP); [303493/2016-0] (CNPq); The authors acknowledge financial support from NIEHS/Superfund Research Program [R01ES002710, P42ES004699, ES025598-01A1, 1K99ES024806]; F.G.H laboratory is funded by NIH [R01DK090492, R01DK095359]; A.B is funded by NIH/NIDDK [R00DK100736]. Authors A.B., F.G.H, B.I., S.K.L, and B.D.H are co-inventors on patents related to sEH
Footnotes
DR. MARCELO HENRIQUE NAPIMOGA (Orcid ID : 0000-0003-4472-365X)
References
- 1.Gemmell E, Yamazaki K, Seymour GJ. Destructive periodontitis lesions are determined by the nature of the lymphocytic response. Crit Rev Oral Biol Med. 2002;13(1):17–34. doi: 10.1177/154411130201300104. [DOI] [PubMed] [Google Scholar]
- 2.Larsen BT, Miura H, Hatoum OA, Gutterman, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BKCa channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids (EETs) on cellular function. Am J Physiol. 2006;292:C96–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 4.Kodani SD, Hammock BD. The 2014 Bernard B. Brodie award lecture-epoxide hydrolases: drug metabolism to therapeutics for chronic pain. Drug Metabol Dispos. 2015 May;43(5):788–802. doi: 10.1124/dmd.115.063339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Node K, Huo Y, Ruan X, et al. Antiinflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao G, Tu L, Li X, et al. Delivery of AAV2-CYP2J2 protects remnant kidney in the 5/6-nephrectomized rat via inhibition of apoptosis and fibrosis. Hum Gene Ther. 2012;23(7):688–99. doi: 10.1089/hum.2011.135. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Yang AL, Liao J, et al. Soluble epoxide hydrolase gene deficiency or inhibition attenuates chronic active inflammatory bowel disease in IL-10(-/-) mice. Dig Dis Sci. 2012;57:2580–2591. doi: 10.1007/s10620-012-2217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris TR, Bettaieb A, Kodani S, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286:102–111. doi: 10.1016/j.taap.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goswami SK, Wan D, Yang J, et al. Anti-Ulcer Efficacy of Soluble Epoxide Hydrolase Inhibitor TPPU on Diclofenac-Induced Intestinal Ulcers. J Pharmacol Exp Ther. 2016;357(3):529–536. doi: 10.1124/jpet.116.232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trindade-da-Silva CA, Bettaieb A, Napimoga MH, et al. Soluble Epoxide Hydrolase Pharmacological Inhibition Decreases Alveolar Bone Loss by Modulating Host Inflammatory Response, RANK-Related Signaling, Endoplasmic Reticulum Stress, and Apoptosis. J Pharmacol Exp Ther. 2017;361(3):408–416. doi: 10.1124/jpet.116.238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napimoga MH, Clemente-Napimoga JT, Macedo CG, et al. Quercetin inhibits inflammatory bone resorption in a mouse periodontitis model. J Nat Prod. 2013;76:2316–2321. doi: 10.1021/np400691n. [DOI] [PubMed] [Google Scholar]
- 12.Bettaieb A, Nagata N, AbouBechara D, et al. Soluble epoxide hydrolase deficiency or inhibition attenuates diet-induced endoplasmic reticulum stress in liver and adipose tissue. J Biol Chem. 2013;288(20):14189–99. doi: 10.1074/jbc.M113.458414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker PJ. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000;2(10):1181–92. doi: 10.1016/s1286-4579(00)01272-7. [DOI] [PubMed] [Google Scholar]
- 14.Bettaieb A, Chahed S, Bachaalany S, Griffey S, Hammock BD, Haj FG. Soluble Epoxide Hydrolase Pharmacological Inhibition Ameliorates Experimental Acute Pancreatitis in Mice. Mol Pharmacol. 2015;88(2):281–90. doi: 10.1124/mol.114.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanella L, Canestraro M, Lee CR, Cao J, Zeldin DC, Schwartzman ML, et al. Soluble epoxide hydrolase null mice exhibit female and male differences in regulation of vascular homeostasis. Prostaglandins Other Lipid Mediat. 2015;120:139–47. doi: 10.1016/j.prostaglandins.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahingur SE, Xia XJ, Alamgir S, Honma K, Sharma A, Schenkein HA. DNA from Porphyromonas gingivalis and Tannerella forsythia induce cytokine production in human monocytic cell lines. Mol Oral Microbiol. 2010;25(2):123–35. doi: 10.1111/j.2041-1014.2009.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montebugnoli L, Servidio D, Miaton RA, Prati C, Tricoci P, Melloni C, et al. Periodontal health improves systemic inflammatory and haemostatic status in subjects with coronary heart disease. J Clin Periodontol. 2005;32(2):188–92. doi: 10.1111/j.1600-051X.2005.00641.x. [DOI] [PubMed] [Google Scholar]
- 19.Holanda K, Lucena-Araujo AR, Quintas A, et al. Mannose-binding lectin 2 (MBL2) gene polymorphisms do not influence frequency of infections in chronic lymphocytic leukemia patients. Rev Bras Hematol Hemoter. 2014;36(1):29–34. doi: 10.5581/1516-8484.20140010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maffei G, Brouwer N, Dolman KM, van der Velden U, Roos D, Loos BG. Plasma levels of mannan-binding lectin in relation to periodontitis and smoking. J Periodontol. 2005;76(11):1881–9. doi: 10.1902/jop.2005.76.11.1881. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R. Toll-like receptors and innate immunity. Nature Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 22.Jin MS, Kim SE, Heo JY, et al. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto C, Oda T, Yokoyama S, Tominari T, Hirata M, Miyaura C, et al. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem Biophys Res Commun. 2012;428(1):110–5. doi: 10.1016/j.bbrc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Samarajiwa SA, Wilson W, Hertzog PJ. The interferons: characterization and application Type I interferons: genetics and structure In Meager A. Weinheim: Wiley-VCH; 2006. pp. 3–34. [Google Scholar]
- 25.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Zadeh HH, Nichols FC, Miyasaki KT. The role of the cell-mediated immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontitis. Periodontol 2000. 1999;20:239–288. doi: 10.1111/j.1600-0757.1999.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 27.Baker PJ, Dixon M, Evans RT, Dufour L, Johnson E, Roopenian DC. CD4 (+) T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect Immun. 1999;67:2804–2809. doi: 10.1128/iai.67.6.2804-2809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Górska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003;30:1046–1052. doi: 10.1046/j.0303-6979.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhu XQ, Lu W, Chen Y, et al. Effects of Porphyromonas gingivalis Lipopolysaccharide Tolerized Monocytes on Inflammatory Responses in Neutrophils. PLoS One. 2016;11(8):e0161482. doi: 10.1371/journal.pone.0161482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herbert BA, Steinkamp HM, Gaestel M, Kirkwood KL. Mitogen-Activated Protein Kinase 2 Signaling Shapes Macrophage Plasticity in Aggregatibacter actinomycetemcomitans-Induced Bone Loss. Infect Immun. 2016;85(1) doi: 10.1128/IAI.00552-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito T, Carson WF, 4th, Cavassani KA, Connett JM, Kunkel SL. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res. 2011;317(5):613–619. doi: 10.1016/j.yexcr.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osamu Y, Kouji M. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]
- 34.Soler D, Chapman TR, Poisson LR, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177(10):6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 35.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 36.Cardoso CR, Garlet GP, Moreira AP, Junior WM, Rossi MA, Silva JS. Characterization of CD4+ CD25+ natural regulatory T cells in the inflammatory infiltrate of human chronic periodontitis. J Leuk Biol. 2008;84:311–318. doi: 10.1189/jlb.0108014. [DOI] [PubMed] [Google Scholar]
- 37.Napimoga MH, da Silva CA, Carregaro V, et al. Exogenous administration of 15d-PGJ2-loaded nanocapsules inhibits bone resorption in a mouse periodontitis model. J Immunol. 2012;189:1043–1052. doi: 10.4049/jimmunol.1200730. [DOI] [PubMed] [Google Scholar]
- 38.Das J, Chen CH, Yang L, Cohn L, Ray P, Ray A. A critical role for NF-κB in GATA3 expression and TH2 differentiation in allergic airway inflammation. Nature Immunol. 2001;2(1):45–50. doi: 10.1038/83158. [DOI] [PubMed] [Google Scholar]
- 39.Tindemans I, Serafini N, Di Santo JP, Hendriks RW. GATA-3 function in innate and adaptive immunity. Immunity. 2014;41(2):191–206. doi: 10.1016/j.immuni.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Niehues T, Perez-Becker R, Schuetz C. More than just SCID—the phenotypic range of combined immunodeficiencies associated with mutations in the recombinase activating genes (RAG) 1 and 2. Clin Immunol. 2010;135(2):183–192. doi: 10.1016/j.clim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Yuan J, Pan Y, et al. T cell CD40LG gene expression and the production of IgG by autologous B cells in systemic lupus erythematosus. Clin Immunol. 2009;132(3):362–370. doi: 10.1016/j.clim.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhen A, Krutzik SR, Levin BR, Kasparian S, Zack JA, Kitchen SG. CD4 ligation on human blood monocytes triggers macrophage differentiation and enhances HIV infection. J Virol. 2014;88(17):9934–9946. doi: 10.1128/JVI.00616-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vernal R, Dutzan N, Hernández M, et al. High expression levels of receptor activator of nuclear factor-kappa B ligand associated with human chronic periodontitis are mainly secreted by CD4 T lymphocytes. J Periodontol. 2006;77:1772–1780. doi: 10.1902/jop.2006.050376. [DOI] [PubMed] [Google Scholar]
- 44.Kawai T, Matsuyama T, Hosokawa Y, et al. B and T Lymphocytes Are the Primary Sources of RANKL in the Bone Resorptive Lesion of Periodontal Disease. Am J Pathol. 2006;169(3):987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarry CR, Martinez EF, Peruzzo DC, et al. Expression of SOFAT by T- and B-lineage cells may contribute to bone loss. Mol Med Rep. 2016;13(5):4252–8. doi: 10.3892/mmr.2016.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thusberg J, Vihinen M. The structural basis of hyper IgM deficiency–CD40L mutations. Protein Eng Des Sel. 2007;20(3):133–141. doi: 10.1093/protein/gzm004. [DOI] [PubMed] [Google Scholar]
- 47.Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 48.Takatsu, Kiyoshi Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(8):463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–638. [PubMed] [Google Scholar]
- 50.Maharjan AS, Roife D, Brazill D, Gomer RH. Serum amyloid P inhibits granulocyte adhesion. Fibrogenesis Tissue Repair. 2013;6:2. doi: 10.1186/1755-1536-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korman BD, Kastner DL, Gregersen PK, Remmers EF. STAT4: genetics, mechanisms, and implications for autoimmunity. Curr Allergy Asthma Rep. 2008;8(5):398–403. doi: 10.1007/s11882-008-0077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenzel J, Peters B, Zahn S, et al. Gene expression profiling of lichen planus reflects CXCL9+-mediated inflammation and distinguishes this disease from atopic dermatitis and psoriasis. J Invest Dermatol. 2008;128(1):67–78. doi: 10.1038/sj.jid.5700945. [DOI] [PubMed] [Google Scholar]


