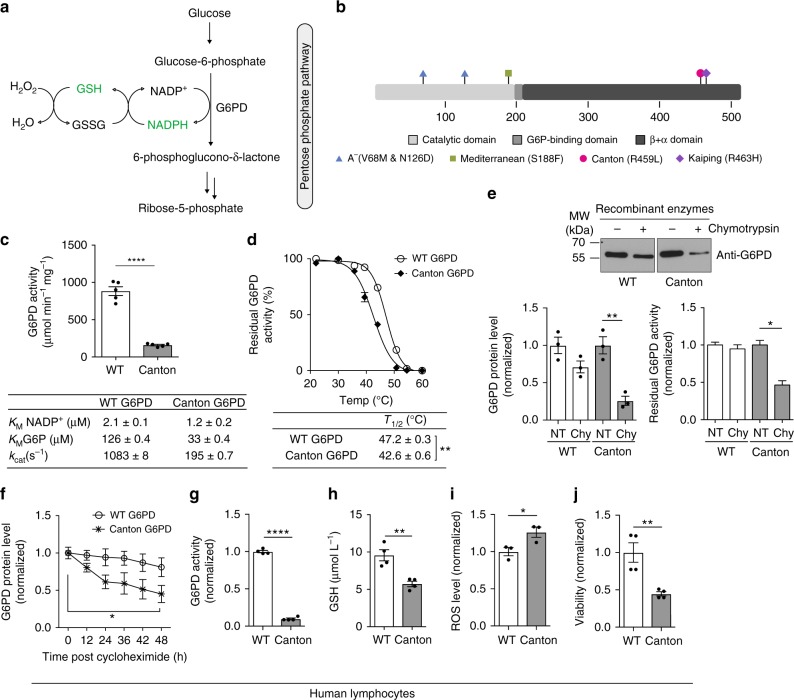
Fig. 1.
Canton G6PD (R459L) variant is biochemically different from WT G6PD. a Enzymatic scheme of G6PD activity. b A linear map of G6PD domain structure with most common variants indicated. c Catalytic activity of recombinant WT G6PD and Canton G6PD enzymes with kinetic parameters (n = 5, ****p < 0.0001). d Thermostability of WT G6PD and Canton G6PD enzyme (n = 3, **p = 0.003). e G6PD protein levels and residual G6PD activity (normalized to NT (no treatment) of each enzyme) after incubation with chymotrypsin for 1 h (n = 3 for protein level assay, **p = 0.0046; n = 2 for enzyme assay, *p = 0.024). f Protein stability assessment with cycloheximide treatment (50 μg mL−1), blocking de novo protein biosynthesis, in lymphocytes derived from corresponding subjects (n = 3, *p = 0.013). Protein levels were normalized to the level of each enzyme at 0 h (no treatment). g G6PD activity was lower in cell lysates with Canton variant (n = 4, ****p < 0.0001). h, i, j Lymphocytes with Canton variant generated less GSH and more reactive oxygen species (ROS) and were less viable (n = 4, (n = 3 for Fig. 1i), *p < 0.05, **p < 0.01). Error bars represent mean ± SEM. Statistical differences were calculated by two-tailed unpaired Student’s t-test. NT: no treatment, WT: wild-type, Chy: chymotrypsin