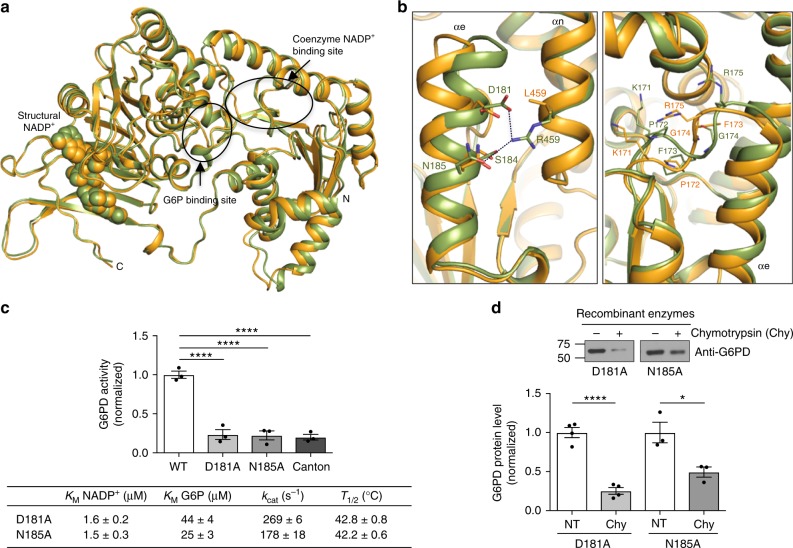
Fig. 2.
Canton mutation (R459L) loses essential inter–helical interactions. a Structural overlay of WT G6PD (green) and Canton variant (orange). Structural NADP+ is shown as spheres, and arrows and circles indicate G6P and catalytic NADP+-binding sites (G6P and catalytic NADP+ were not observed in our structures). b (Left) Inter-helical interactions through R459 on the αn helix in WT G6PD and side chains of D181 and N185 on the adjacent helix (αe). (Right) Canton mutation loses such interactions, leading to displacements of the helix (αe) and a loop containing K171, P172, F173, G174 and R175 that precedes the helix. c, d Mutations of R459-interacting residues on the αe helix showed Canton mutation-like activity and thermostability (n = 3, ****p < 0.0001, one-way ANOVA) and were also susceptible to chymotrypsin treatment (n = 4 for D181A, ****p < 0.0001; n = 3 for N185A, *p = 0.026, two-tailed unpaired Student’s t-test). Error bars represent mean ± SEM. NT no treatment; WT: wild-type; Chy: chymotrypsin