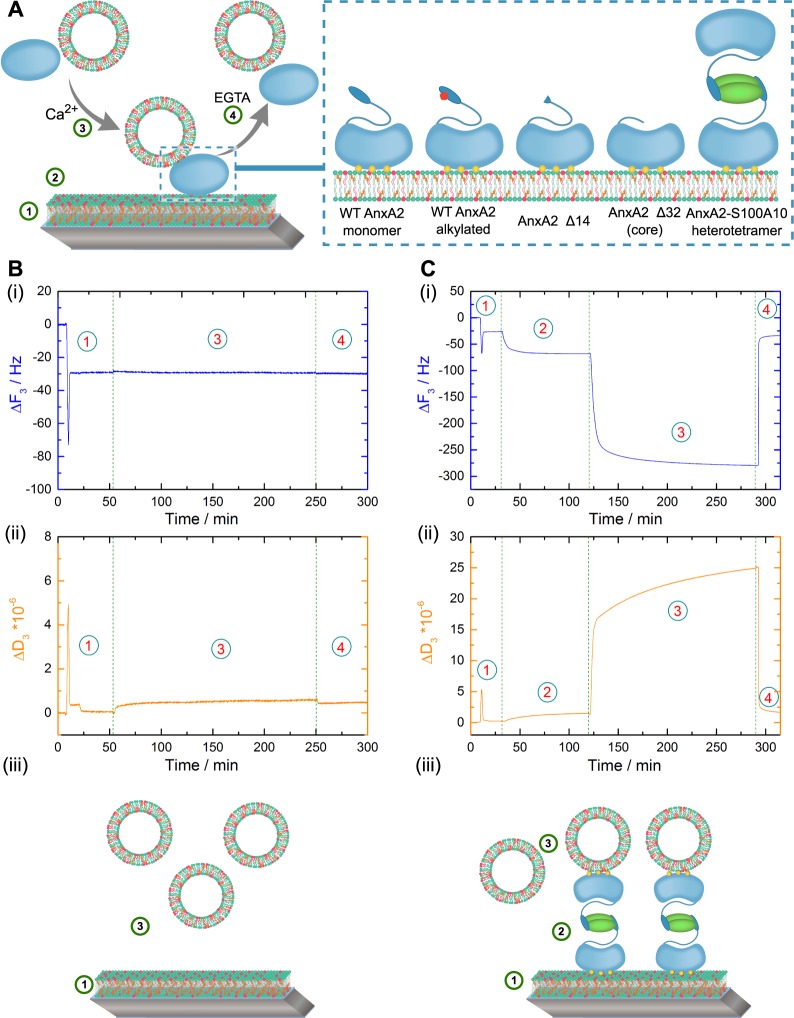
Figure 1.
(A) Schematic model of the sequential lipid and protein additions in the quartz crystal microbalance with dissipation (QCM-D) setup. (1) Solid supported lipid bilayer (SLB) formation following SUV adsorption and rupture, (2) addition and adsorption of protein derivatives in the presence of 250 µM Ca2+ (the AnxA2 derivates used are depicted in the inset), (3) application of second membrane surface presented in the form of LUVs, (4) removal of all Ca2+-dependently bound protein and/or lipid by perfusion with EGTA-containing buffer. Inset: Schematic representation of the AnxA2 derivatives used in this study: (i) WT AnxA2, (ii) alkylated AnxA2, deletion mutants (iii) AnxA2 Δ32 and (iv) AnxA2 Δ14, and (v) the heterotetrameric (AnxA2)2-(S100A10)2 complex (A2t). (B) Addition of LUVs to a SLB layer in the absence of protein. Shown are QCM-D recordings, frequency (i) and dissipation changes (ii), revealing the formation of an SLB layer following SUV addition and rupture (step 1) and the absence of any frequency or dissipation shift upon addition of the second vesicle population in the form of LUVs (step 3). (C) Membrane bridging/linking by the heterotetrameric A2t complex. Time dependent frequency (i) and dissipation (ii) monitoring of SLB formation (step 1), Ca2+ (250 µM) dependent binding of A2t to the SLB layer containing negatively charged phospholipids and cholesterol (step 2), interaction with a secondary vesicle population (LUVs) in the presence of 250 µM Ca2+ (step 3) and complete reversibility of the protein and LUV adsorption by addition of Ca2+ chelating EGTA buffer (step 4). Models depicting the respective experimental setup are shown below the QCM-D recordings (iii). Shown are representative examples of typical experiments carried out at least n = 20 (B) or n = 12 (C) independent times with at least three different protein batches and vesicle preparations.