Abstract
Purpose:
To investigate the use of proton pump inhibitors (PPIs) and the risk of pancreatic cancer.
Methods:
A nested case–control analysis was conducted. Patients with pancreas cancer were matched with controls by propensity score. Univariate and multivariate logistic regression models were used to determine whether PPIs use affected the risk of pancreas cancer. Dose effect was analyzed based on the cumulative defined daily dose (DDD), which was calculated using the total supply of PPIs to individual patients in terms of days and quantity.
Results:
A total of 1087 patients with pancreas cancer were matched with 1087 control patients from the database. The overall adjusted odds ratio (OR) of PPI use associated with pancreas cancer was 1.69 (95% confidence interval [CI], 1.44-2.05). Dose analysis by cumulative DDD, based on all types of PPI combined, revealed a lower adjusted OR of 0.92 (95% CI, 0.64-1.33) for those on <30 cumulative DDD compared with those on ≥150 cumulative DDD, whose adjusted OR was 2.19 (95% CI, 1.68-2.85). Compared with PPI nonusers, the risks of pancreas cancer were: OR 0.89 (95% CI, 0.62-1.27) for patients using PPI <30 days and 2.22 (95% CI, 1.68-2.94) for ≥150 days.
Conclusions:
Risk of pancreas cancer was associated with PPI use in patients with peptic ulcer diseases or gastroesophageal reflux disease.
Keywords: National Health Insurance Research database, pancreas cancer, proton pump inhibitors, nested case–control, population-based
Introduction
In the past few decades, proton pump inhibitors (PPIs) have been very effective in maintaining symptomatic and endoscopic remission of acid peptic disorders, such as gastroesophageal reflux and peptic ulcer.1,2 A number of recent studies have investigated the pleiotropic effects of PPIs, including immunomodulation, anti-inflammation, and anticancer or carcinogenesis, and the results have raised concerns about the side effects of PPIs.3,4
Pancreatic adenocarcinoma is a deadly cancer with a poor response to chemotherapy and a dismal outcome.5 Risk factors related to pancreas cancer include chronic pancreatitis, smoking, diabetes, and obesity.6,7 Carcinogenesis in the pancreas remains poorly understood. Thus, there is an urgent need to identify additional risk factors that could enable a better understanding of the roles played by various drugs in the development of pancreas cancer. These data could prove valuable for preventive modalities such as screening and the development of chemoprevention agents.
The effects of drugs on the risk of developing pancreas cancer, as well as their preventive effects, are an important area of cancer research. Aspirin, metformin, statins, β-blockers, and bisphosphonates are known to biologically inhibit pancreatic neoplasia.8 Most studies concerning aspirin and its role in pancreas cancer have shown that it reduces the risk, and high-dose aspirin, rather than low-dose aspirin, appeared to be associated with decreased risk for pancreatic cancer.9 Previous reports on the association of PPI use and pancreatic cancer have yielded conflicting findings.10,11 Relatively little research has been conducted on PPI use and pancreas cancer, particularly in Asia. Chien et al demonstrated that the use of PPI was associated with periampullary tumor in a population-based study conducted in Taiwan. Though pancreatic head cancer was included in the analysis, the main focus of the study was the periduodenum.12 Concerning hepatic cytochrome p450 enzyme, people of Asian descent are more likely to be slow metabolizers of PPIs than people in other ethnic groups.13 We conducted a nested case–control study using a population-based data set to determine the risk of pancreas cancer in patients taking PPIs.
Materials and Methods
Data Source
Taiwan’s universal, single-payer National Health Insurance (NHI) program was established in 1995 and offers comprehensive medical coverage for over 99% of the population (approximately 23.5 million in 2018).14 The National Health Research Institutes is in charge of the entire insurance claims database, or the NHIRD, which contains registration files and original medical claims data of all beneficiaries. The data are encrypted with unique personal identifications in accordance with strict privacy protocols. The NHIRD has been used extensively in many epidemiologic studies conducted in Taiwan. The diagnostic codes used in NHIRD are based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Patients on the Registry of Catastrophic Illness Database (RCIPD), a subset of the NHIRD, comprised the case group. The RCIPD includes data from insured residents with severe diseases, as defined by the NHI program, such as malignancies, transplant, or autoimmune diseases. The longitudinal health insurance database (LHID2000) is a data set containing the original claims data of 1 000 000 individuals randomly sampled from the “2000 Registry for Beneficiaries” database, which is a part of the NHIRD. The LHID2000 contains the registration data of everyone who was a beneficiary in the NHI program during the period 1996 to 2000. There was no significant difference in gender distribution between patients in the LHID2000 and those in the original NHIRD.
Ethics Statement
The NHIRD encrypts patients’ personal information to protect their privacy and provides researchers with anonymous identification numbers associated with relevant claims information, including sex, date of birth, medical services received, and prescriptions. Therefore, patient consent is not required to access the NHIRD. This study was approved by the institutional review board of China Medical University (CMUH104-REC2-115-CR2), which also waived the requirement for patient consent.
Data Availability Statement
The data set used in this study is managed by Taiwan’s Ministry of Health and Welfare (MOHW). Researchers can request access to this data set by submitting an application form to the MOHW. Please contact the MOHW (email: stcarolwu@mohw.gov.tw) for further details (address: No. 488, Sec 6, Zhongxiao E. Rd, Nangang Dist, Taipei City 115, Taiwan, ROC). Phone: +886-2-8590-6848). All relevant data analyzed in this article are presented herein.
Study Populations and Disease Codes
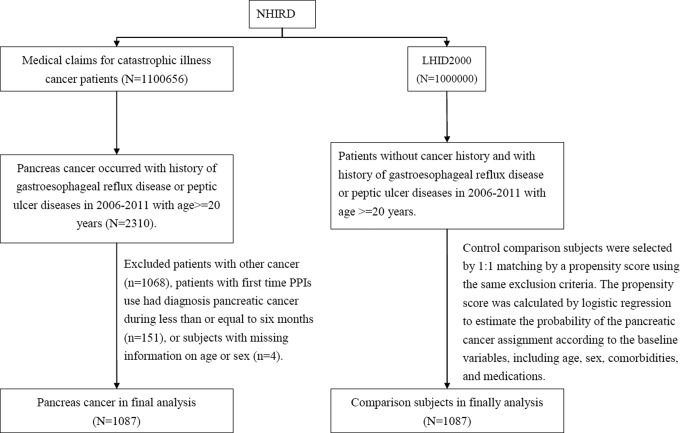
Patients diagnosed with gastroesophageal reflux disease (GERD; ICD-9 codes 530.81, 530.11) or peptic ulcer diseases (ICD-9 codes 531-534) formed the base population. We also included patients with newly diagnosed pancreatic cancer (ICD-9-CM 157) between January 1, 2006, and December 31, 2011, from the RCIPD. The date of diagnosis of pancreatic cancer was defined as the index date. Patients were excluded if they had other malignancy (ICD-9-CM codes 140-208) before the index date, were younger than 20 years, or had missing information on age or sex. Control patients in the nonpancreatic cancer group were randomly selected from the patients with GERD or peptic ulcer disease without cancer. The control patients were randomly assigned an index date within the same index year of the matched pancreatic cancer cases. We also excluded patients who were diagnosed with pancreatic cancer during the 6-month period following first-time use of PPIs. To reduce selection bias, propensity score was applied to select the 2 groups with and without pancreatic cancer in a 1:1 ratio. The propensity score was calculated using logistic regression to estimate the probability of developing pancreatic cancer based on the baseline variables including gender, age, year of pancreatic cancer diagnosis, medications (H2-receptor antagonist [H2RA], aspirin, metformin), and comorbidities (gastric polyp, gastritis, cirrhosis, diabetes, chronic pancreatitis, hepatitis B infection, hepatitis C infection, inflammatory bowel disease, biliary tract disease, stroke, coronary arterial disease, chronic obstructive pulmonary disease, and alcohol-related illness). In order to avoid possible bias caused by differences in the observation period, each patient was only retrospectively observed for 5 years. A total of 1087 cases with pancreatic cancer and 1087 controls without pancreatic cancer were included in the final analysis (Figure 1).
Figure 1.
Study flow diagram. CCA indicates cholangiocarcinoma; GERD, gastroesophageal reflux disease; LHID2000, longitudinal health insurance database; NHIRD, National Health Insurance Research Database; PUD, peptic ulcer disease.
Exposure to PPIs
The primary exposures of interest were PPI use since the date of entry into the database until 2 years prior to the index date. The PPI data were defined according to the Anatomical, Therapeutic and Chemical/Defined Daily Dose (ATC/DDD) system of the World Health Organization Collaborating Centre for Drug Statistics and Methodology. Patients with claims for PPIs including omeprazole (ATC A02BC01), pantoprazole (ATC A02BC02), lansoprazole (ATC A02BC03), rabeprazole (ATC A02BC04), and esomeprazole (ATC A02BC05) before the index date were assigned to the exposure to PPI group. The cumulative DDD of each type of PPIs prescribed for the pancreatic cancer group and control group was determined. The DDD is a unit that is used to measure a prescribed amount of drug and was defined according to the abovementioned ATC/DDD system. This is the assumed average maintenance dose per day of a drug consumed by the patient.15 All types of PPI were compared based on the same standard according to the following formula: total amount of drug/amount of drug in a DDD = number of DDDs. Cumulative DDD was estimated as the sum of the dispensed DDDs of any PPI. The overall cumulative duration of PPI was also calculated by summing the daily supply of each type of PPI before the index date.
Statistical Analysis
All analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc, Cary, New Carolina). The χ2 test was used to determine the difference in categorical variables between the pancreatic cancer and control groups, while the 2-sample Student t test was used to determine the differences in continuous variables. Univariable and multivariable logistic regression were used to estimate the effect of PPI treatment and comorbidities on the risk of pancreatic cancer, as indicated by the odds ratio (OR) with 95% confidence interval (CI). Results were considered statistically significant when 2-tailed P values were less than .05.
Results
Demographics and Characteristics of Study Patients
The demographic data of both study groups are shown in Table 1. There were no statistically significant differences in the distributions of gender, age, H2RA medications, aspirin, and metformin, and comorbidities. The pancreatic cancer groups had a higher rate of chronic pancreatitis. The mean age of the pancreatic cancer group was 67.4 (±11.5) and compared with 68.3 (±13.6) years in the controls. Patients with pancreatic cancer tended to have a higher prevalence of PPI use compared with the control group (P values <.001).
Table 1.
Baseline Characteristics of Patients With and Without Pancreatic Cancer.a
| Pancreatic Cancer | P Valueb | ||||
|---|---|---|---|---|---|
| No, N = 1087 | Yes, N = 1087 | ||||
| n | % | n | % | ||
| Gender | .60 | ||||
| Women | 425 | 39.1 | 437 | 40.2 | |
| Men | 662 | 60.9 | 650 | 59.8 | |
| Age (year) | |||||
| Mean (SD)b | 68.3 | 13.6 | 67.4 | 11.5 | .09 |
| Medications | |||||
| PPI | 320 | 29.4 | 454 | 41.8 | <.001 |
| H2RA | 908 | 83.5 | 934 | 85.9 | .12 |
| Aspirin | 603 | 55.5 | 602 | 55.4 | .97 |
| Metformin | 331 | 30.5 | 342 | 31.5 | .61 |
| Baseline comorbidities | |||||
| Gastritis | 757 | 69.6 | 739 | 68.0 | .40 |
| Acute pancreatitis | 126 | 11.6 | 155 | 14.3 | .06 |
| Chronic pancreatitis | 67 | 6.16 | 93 | 8.56 | .03 |
| Diabetes | 344 | 31.7 | 340 | 31.3 | .85 |
| Hypertension | 733 | 67.4 | 699 | 64.3 | .12 |
| COPD | 482 | 44.3 | 477 | 43.9 | .83 |
| CAD | 432 | 39.7 | 416 | 38.3 | .48 |
| Heart failure | 91 | 8.37 | 89 | 8.19 | .88 |
| Cirrhosis | 558 | 51.3 | 550 | 50.6 | .73 |
| Hepatitis B infection | 108 | 9.94 | 115 | 10.6 | .62 |
| Hepatitis C infection | 78 | 7.18 | 75 | 6.90 | .80 |
| Inflammatory bowel disease | 55 | 5.06 | 46 | 4.23 | .36 |
| Biliary tract disease | 278 | 25.6 | 283 | 26.0 | .81 |
| Stroke | 138 | 12.7 | 120 | 11.0 | .23 |
| Gastric polyp | 12 | 1.10 | 17 | 1.56 | .35 |
| Alcohol-related disease | 115 | 10.6 | 108 | 9.94 | .62 |
Abbreviations: CAD, coronary arterial disease; COPD, chronic obstructive pulmonary disease; H2RA, H2-receptor antagonist; PPI, proton pump inhibitor; SD, standard deviation.
aData are presented as the number of patients in each group, with percentages given in parentheses.
bχ2test and t test comparing patients with and without pancreatic cancer.
Risk of Pancreatic Cancer Associated With PPIs and Covariates
The ORs of estimated pancreas cancer risk based on PPI use are shown in Table 2. The PPI use was associated with an increased risk of pancreas cancer and the association was statistically significant (adjusted OR [aOR] = 1.69, 95% CI, 1.42-2.03]. A comparison of the 2 groups based on age revealed that patients aged 65 to 74 years had a higher risk for pancreatic cancer (aOR = 1.25, 95% CI, 1.01-1.55). The crude ORs were not significant for other medications, including H2RA, aspirin, and metformin, or for comorbidities, except for chronic pancreatitis.
Table 2.
Odds Ratios and 95% Confidence Intervals of Pancreatic Cancer Associated With Proton Pump Inhibitor and Covariates.
| Crude | Adjusteda | |||
|---|---|---|---|---|
| Variable | OR | 95% CI | OR | 95% CI |
| Gender | ||||
| Women | 1 | Reference | 1 | Reference |
| Men | 0.96 | 0.80, 1.13 | ||
| Age (years) | ||||
| ≤64 | 1.17 | 0.96, 1.43 | 1.15 | 0.93, 1.41 |
| 65-74 | 1.26 | 1.02, 1.56b | 1.25 | 1.01, 1.55b |
| ≥75 | 1 | Reference | 1 | Reference |
| Medications | ||||
| PPI | 1.72 | 1.44, 2.05c | 1.69 | 1.42, 2.03c |
| H2RA | 1.20 | 0.95, 1.52 | ||
| Aspirin | 1.00 | 0.84, 1.18 | ||
| Metformin | 1.05 | 0.87, 1.26 | ||
| Baseline comorbidities | ||||
| Gastritis | 0.93 | 0.77, 1.10 | ||
| Acute pancreatitis | 1.27 | 0.99, 1.63 | ||
| Chronic pancreatitis | 1.42 | 1.03, 1.97b | 1.26 | 0.90, 1.76 |
| Diabetes | 0.98 | 0.82, 1.18 | ||
| Hypertension | 0.87 | 0.73, 1.04 | ||
| COPD | 0.98 | 0.83, 1.16 | ||
| CAD | 0.94 | 0.79, 1.12 | ||
| Heart failure | 0.98 | 0.72, 1.32 | ||
| Cirrhosis | 0.97 | 0.82, 1.15 | ||
| Hepatitis B infection | 1.07 | 0.82, 1.42 | ||
| Hepatitis C infection | 0.96 | 0.69, 1.33 | ||
| Inflammatory bowel disease | 0.83 | 0.56, 1.24 | ||
| Biliary tract disease | 1.02 | 0.85, 1.24 | ||
| Stroke | 0.85 | 0.66, 1.11 | ||
| Gastric polyp | 1.42 | 0.68, 3.00 | ||
| Alcohol-related disease | 0.93 | 0.71, 1.23 | ||
Abbreviations: CAD, coronary arterial disease; COPD, chronic obstructive pulmonary disease; H2RA, H2-receptor antagonist; PPI, proton pump inhibitor.
a Adjusted for age group and chronic pancreatitis.
b P < .05.
c P < .001.
Dosage of PPIs and Risk of Pancreatic Cancer
Dose analysis by cumulative DDD was performed. Table 3 shows the dose–response relationship between PPI use and pancreatic cancer risk, compared with controls. For all types of PPIs combined, the OR increased was 1.68 (95% CI, 1.14-2.49) for patients on <30 cumulative DDD compared with 2.19 (95% CI, 2.68-2.85) for those on ≥150 cumulative DDD. Compared with controls, the risks of pancreatic cancer were: OR 0.89 (95% CI, 0.62-1.27) for use of PPI <30 days and 2.22 (95% CI, 1.68-2.94) for ≥150 days.
Table 3.
Odds Ratio and 95% Confidence Intervals of Pancreatic Cancer Associated With Cumulative DDD Dose and Cumulative Use Day of Proton Pump Inhibitors.
| Case Number/Control Number | Crude Odds Ratio | 95% CI | Adjusted odds ratioa | 95% CI | |
|---|---|---|---|---|---|
| Nonuse of PPI | 633/767 | 1.00 | Reference | 1.00 | Reference |
| PPIb | |||||
| <30 DDD | 55/72 | 0.93 | 0.64, 1.34 | 0.92 | 0.64, 1.33 |
| 30-65 DDD | 64/46 | 1.69 | 1.14, 2.50c | 1.68 | 1.14, 2.49c |
| 65-150 DDD | 148/100 | 1.79 | 1.36, 2.36d | 1.77 | 1.34, 2.33d |
| ≥150 DDD | 187/102 | 2.22 | 1.71, 2.89d | 2.19 | 1.68, 2.85d |
| P for trend | <0.001 | <0.001 | |||
| PPIb | |||||
| <30 day | 58/79 | 0.89 | 0.62, 1.27 | 0.89 | 0.62, 1.27 |
| 30-65 day | 84/52 | 1.96 | 1.36, 2.81d | 1.94 | 1.35, 2.78d |
| 65-150 day | 144/99 | 1.76 | 1.34, 2.32d | 1.75 | 1.33, 2.31d |
| ≥150 day | 168/90 | 2.26 | 1.72, 2.98d | 2.22 | 1.68, 2.94d |
| P for trend | <0.001 | <0.001 |
Abbreviation: DDD, defined daily dose.
a Adjusted for age group and biliary tract disease.
b The cumulative DDD dose is partitioned into 2 segments by third quartile.
c P < .01.
d P < .001.
Table 4 shows cumulative DDD for individual PPIs and the risk of pancreatic cancer. Dose analysis showed the highest risk for patients using <70 cumulative DDD of rabeprazole (aOR = 6.31, 95% CI, 2.41-16.5), followed by <60 cumulative DDD of lansoprazole (aOR = 2.26, 95% CI, 1.45-3.53), ≥60 cumulative DDD of lansoprazole (aOR = 2.59, 95% CI, 1.67-4.03), ≥50 cumulative DDD of pantoprazole (aOR = 1.98, 95% CI, 1.13-3.34), and ≥85 cumulative DDD of esomeprazole (aOR = 1.76, 95% CI, 1.11-2.79; Table 4).
Table 4.
Odds Ratio and 95% Confidence Intervals of Pancreatic Cancer Associated With Cumulative DDD Dose of Individual Proton Pump Inhibitors.
| Case Number/Control Number | Crude Odds Ratio | 95% CI | Adjusted Odds Ratioa | 95% CI | |
|---|---|---|---|---|---|
| Nonuse of PPI | 633/767 | 1.00 | Reference | 1.00 | Reference |
| Omeprazoleb | |||||
| <40 DDD | 69/68 | 1.23 | 0.87, 1.75 | 1.24 | 0.87, 1.76 |
| ≥40 DDD | 65/54 | 1.46 | 1.00, 2.12c | 1.46 | 1.00, 2.12 |
| P for trend | <0.001 | <0.001 | |||
| Pantoprazoleb | |||||
| <50 DDD | 30/27 | 1.35 | 0.79, 2.29 | 1.37 | 0.80, 2.34 |
| ≥50 DDD | 34/21 | 1.96 | 1.13, 3.41c | 1.98 | 1.13, 3.44c |
| P for trend | <0.001 | <0.001 | |||
| Lansoprazoleb | |||||
| <60 DDD | 60/32 | 2.27 | 1.46, 3.53d | 2.26 | 1.45, 3.53d |
| ≥60 DDD | 66/31 | 2.58 | 1.66, 4.00d | 2.59 | 1.67, 4.03d |
| P for trend | <0.001 | <0.001 | |||
| Rabeprazoleb | |||||
| <70 DDD | 26/5 | 6.30 | 2.41, 16.5d | 6.31 | 2.41, 16.5d |
| ≥70 DDD | 16/10 | 1.94 | 0.87, 4.30 | 1.93 | .87, 4.29 |
| P for trend | <0.001 | <0.001 | |||
| Esomeprazoleb | |||||
| <85 DDD | 40/39 | 1.24 | 0.79, 1.96 | 1.24 | 0.79, 1.95 |
| ≥85 DDD | 48/33 | 1.76 | 1.12, 2.78c | 1.76 | 1.11, 2.79c |
| P for trend | <0.001 | <0.001 |
Abbreviation: DDD, defined daily dose.
a Adjusted for age group and biliary tract disease.
b The cumulative DDD dose is partitioned into 2 segments by median.
c P < .05.
d P < .001.
Discussion
Our results showed that the use of PPI was associated with the risk of pancreatic cancer (aOR = 1.69, 95% CI, 1.42-2.03). For all types of PPI combined, the OR was 1.68 (95% CI, 1.14-2.49) for patients on <30 cumulative DDD compared with 2.19 (95% CI, 2.68-2.85) for those on ≥150 cumulative DDD. The cumulative DDD of individual PPIs was also associated with risk of pancreatic cancer.
The present study is, to the best of the authors’ knowledge, the largest investigation of the effects of PPIs on risk of pancreatic cancer in an Asian population. A previous report conducted in Europe (United Kingdom) showed that anti-acid treatment (H2RA and PPI) was not associated with risk of pancreatic cancer.10 However, recent data showed that PPI therapy may be associated with pancreatic cancer risk and PPI users recently started on treatment had slightly worse survival.11 The use of PPI was associated with an increased risk of periampullary cancers, including pancreas head cancer, in a population-based study from NHIRD.12 The association of PPI use and pancreatic cancer risk requires further investigation in order to establish the precise nature of the relationship as well as the underlying mechanism. Furthermore, ethnicity appears to play a role as East Asians are known to be slow metabolizers of PPIs as a result of a lower expression of hepatic cytochrome p450.13 Our results demonstrated that PPI use was associated with pancreatic cancer risk in our population-based database.
Gastrin has 2 physiological roles related to meal-stimulated gastric acid secretion and as a trophic hormone for epithelial and enterochromaffin cells.16 Gastrin and its receptors are coexpressed in pancreatic cancer.17,18 Hypergastrinemia has been shown to be associated with carcinogenesis, and thus it is reasonable to postulate that PPI-induced hypergastrinemia could be considered a factor in the development of pancreatic cancer.12,17,19,20 It is possible that gastrin and PPIs interact, resulting in a cellular microenvironment that is more conducive to pancreatic carcinogenesis in susceptible patients. However, the aOR for developing periampullary cancers in patients taking PPIs was 1.35 (95% CIs, 1.16-1.57), and for pancreatic cancer was 1.69 in the present study. Thus, the risk of periampullary cancers and pancreatic cancer in PPI users, although not high, was nonetheless statistically significant. Compared with the risk of developing gastric cancer in patients without GERD or PPI use, the risk of gastric cancer in patients with GERD taking PPIs was also moderate and seemed to be higher than that for pancreatic cancer in our previous report.20,21
To evaluate the potential dose–response relationship, we summed up the doses of PPIs and stratified PPI use into less than 30, 30 to 65, 65 to 150, and more than 150 cumulative DDDs. We demonstrated a statistically significant trend between the dose and the cumulative incidences of pancreatic cancer. Therefore, taken together, our results revealed a significant association between use of PPIs and risk of pancreatic cancer.
This study had several limitations. First, patients’ compliance with medication could not be confirmed in the NHIRD. Second, patients’ lifestyle behaviors, such as alcohol and smoking habits, were not collected in the NHIRD. Third, most patients may have taken more than one type of PPI, so the interaction of different PPIs could not be completely accounted for. In addition, use of over-the-counter PPIs could not be evaluated. Fourth, this study employed a retrospective case–control design and thus it was not possible to definitively establish any cause-effect relationships between use of PPIs and pancreatic cancer. As such, further research using randomized controlled trials is needed to determine whether PPI use causes pancreatic cancer.
Conclusion
The PPI use was associated with the risk of pancreatic cancer in patients taking PPIs for peptic ulcer diseases or GERD. Further investigations with a prospective and randomized controlled study design are warranted to explore the relationship between PPI use and pancreatic cancer risk.
Footnotes
Authors’ Note: Y-.C.P. and C-.H.K. contributed to conceptualization, investigation, resource, supervision, and project administration; Y-.C.P., C-.L.L., W-.Y.H., I-.T.L., H-.Z.Y., C-.S.C., and C-.H.K. contributed to methodology, data curation, validation, and formal analysis and wrote original draft (preparation and visualized, review and editing); C-.L.L. and C-.H.K. contributed to software; and C-.H.K. did funding acquisition.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital (DMR-107-192); Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-); Tseng-Lien Lin Foundation, Taichung, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
ORCID iD: Chia-Hung Kao  http://orcid.org/0000-0002-6368-3676
http://orcid.org/0000-0002-6368-3676
References
- 1. Moayyedi P, Talley NJ. Gastro-oesophageal reflux disease. Lancet. 2006;367(9528):2086–2100. [DOI] [PubMed] [Google Scholar]
- 2. Gralnek IM, Barkun AN, Bardou M. Management of acute bleeding from a peptic ulcer. N Engl J Med. 2008;359(9):928–937. [DOI] [PubMed] [Google Scholar]
- 3. Han YM, Park JM, Kangwan N, et al. Role of proton pump inhibitors in preventing hypergastrinemia-associated carcinogenesis and in antagonizing the trophic effect of gastrin. J Physiol Pharmacol. 2015;66(2):159–167. [PubMed] [Google Scholar]
- 4. Handa O, Yoshida N, Fujita N, et al. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm Res. 2006;55(11):476–480. [DOI] [PubMed] [Google Scholar]
- 5. MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004;5(9):541–549. [DOI] [PubMed] [Google Scholar]
- 6. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. [DOI] [PubMed] [Google Scholar]
- 7. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378(9791):607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amin S, Boffetta P, Lucas AL. The role of common pharmaceutical agents on the prevention and treatment of pancreatic cancer. Gut Liver. 2016;10(5):665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cui XJ, He Q, Zhang JM, Fan HJ, Wen ZF, Qin YR. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43(1):135–140. [DOI] [PubMed] [Google Scholar]
- 10. Bradley MC, Murray LJ, Cantwell MM, Hughes CM. Proton pump inhibitors and histamine-2-receptor antagonists and pancreatic cancer risk: a nested case–control study. Br J Cancer. 2012;106(1):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearns MD, Boursi B, Yang YX. Proton pump inhibitors on pancreatic cancer risk and survival. Cancer Epidemiol. 2017;46:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chien LN, Huang YJ, Shao YH, et al. Proton pump inhibitors and risk of periampullary cancers – a nested case–control study. Int J Cancer. 2016;138(6):1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi S, Klotz U. Proton pump inhibitors: an update of their clinical use and pharmacokinetics. Eur J Clin Pharmacol. 2008;64(10):935–951. [DOI] [PubMed] [Google Scholar]
- 14. NHIR database. Taiwan. 2015. http://nhird.nhri.org.tw/en/index.html. Accessed September 21, 2018.
- 15. WHO International Working Group for Drug Statistics Methodology. Introduction to Drug Utilization Research. Oslo, Norway: World Health Organization; 2003. [Google Scholar]
- 16. Dockray GJ. Clinical endocrinology and metabolism. Gastrin Best Pract Res Clin Endocrinol Metab. 2004;18(4):555–568. [DOI] [PubMed] [Google Scholar]
- 17. Harris JC, Gilliam AD, McKenzie AJ, et al. The biological and therapeutic importance of gastrin gene expression in pancreatic adenocarcinomas. Cancer Res. 2004;64(12):5624–5631. [DOI] [PubMed] [Google Scholar]
- 18. Smith JP, Fonkoua LK, Moody TW. The role of gastrin and CCK receptors in pancreatic cancer and other malignancies. Int J Biol Sci. 2016;12(3):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238(1):15–29. [DOI] [PubMed] [Google Scholar]
- 20. Peng YC, Huang LR, Lin CL, et al. Association between proton pump inhibitors use and risk of gastric cancer in patients with GERD [published online ahead of print February 22, 2018]. Gut. 2018. [DOI] [PubMed] [Google Scholar]
- 21. Wan QY, Wu XT, Li N, Du L, Zhou Y. Long-term proton pump inhibitors use and risk of gastric cancer: a meta-analysis of 926 386 participants [published online ahead of print April 3, 2018]. Gut 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set used in this study is managed by Taiwan’s Ministry of Health and Welfare (MOHW). Researchers can request access to this data set by submitting an application form to the MOHW. Please contact the MOHW (email: stcarolwu@mohw.gov.tw) for further details (address: No. 488, Sec 6, Zhongxiao E. Rd, Nangang Dist, Taipei City 115, Taiwan, ROC). Phone: +886-2-8590-6848). All relevant data analyzed in this article are presented herein.