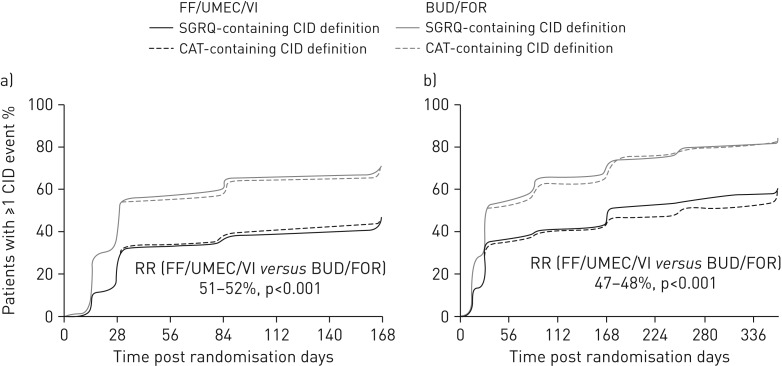
FIGURE 1.
Time to a first composite clinically important deterioration (CID) event in a) the intent-to-treat population (24-week study duration, n=1810) and b) the extension population (52-week study duration, n=430). RR: risk reduction (based on a time-to-first-event analysis using a Cox proportional hazards model); FF: fluticasone furoate; UMEC: umeclidinium; VI: vilanterol; BUD: budesonide; FOR: formoterol; SGRQ: St George's Respiratory Questionnaire; CAT: chronic obstructive pulmonary disease assessment test.