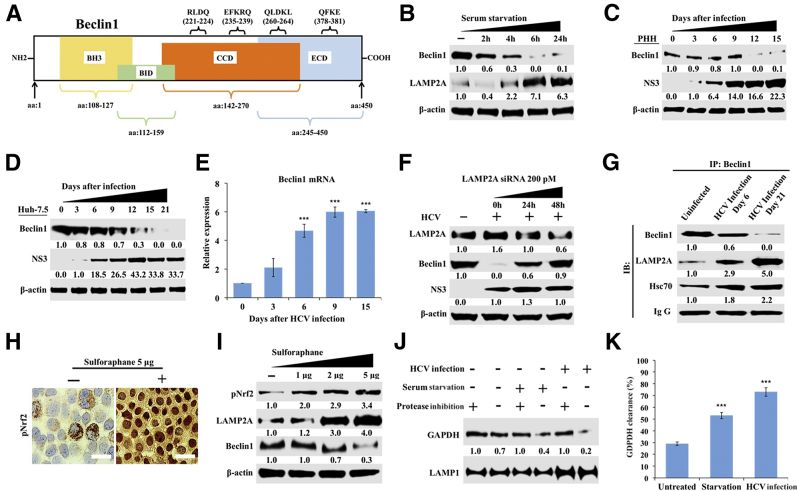
Figure 4.
Chaperone-mediated autophagy (CMA) targets beclin1 degradation in hepatitis C virus (HCV) infection. A: Schematic representation showing the presence of multiple pentapeptide motif (KFERQ-like), CMA motifs, and their location in beclin1 protein sequences. B: Western blot shows beclin1 and lysosome-associated membrane protein 2A (LAMP2A) expression in Huh-7.5 cells after serum starvation. C: Beclin1 expression in HCV-infected primary human hepatocytes (PHHs). D: Beclin1 expression in HCV-infected Huh-7.5 cells. E: Beclin1 mRNA levels in HCV-infected Huh-7.5 cells by real-time quantitative RT-PCR. F: Western blot analysis shows silencing LAMP2A expression restores beclin1 expression without altering HCV replication in infected Huh-7.5 cells. G: Co-immunoprecipitation shows interaction among beclin1, LAMP2A, and heat shock cognate 70 kDa (Hsc70) in uninfected and HCV-infected Huh-7.5 cells at 6 days and 21 days. H: Sulforaphane induces nuclear factor erythroid 2–related factor (Nrf2) activation and its nuclear translocation in uninfected Huh-7.5 cells. I: Western blot analysis demonstrating expression of LAMP2A and beclin1 in uninfected Huh-7.5 cells after treatment with increasing concentrations of sulforaphane. J: CMA functional assay showing lysosomal degradation of recombinant glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the presence and absence of protease inhibitors. K: Quantification of GAPDH bands in Western blot showing amount of GAPDH uptake and degradation during serum starvation and late HCV infection. ∗∗∗P < 0.001 versus day 0. Scale bars = 200 μm.