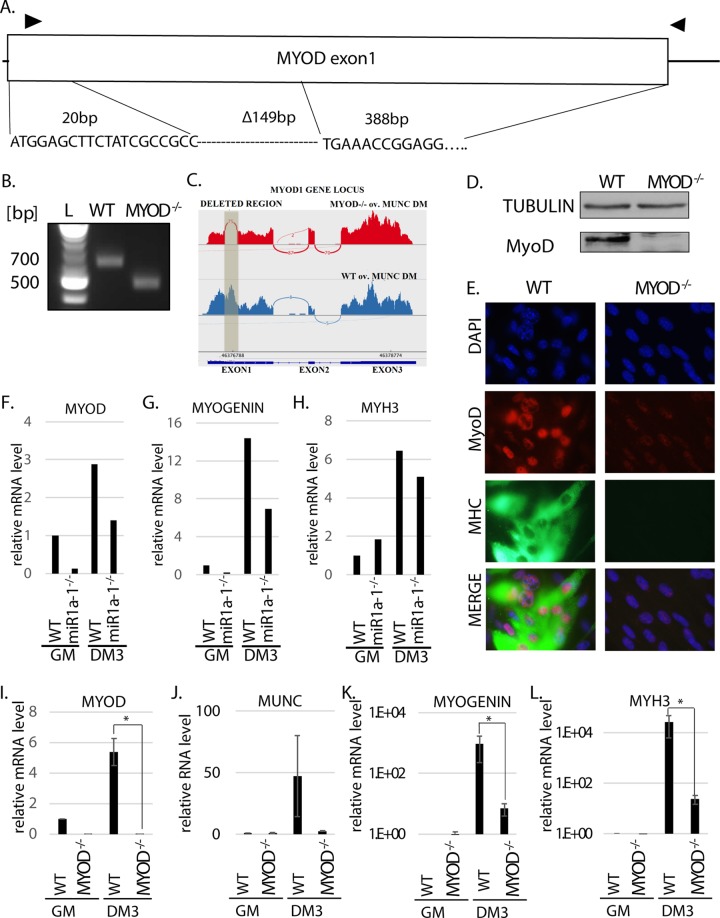
FIG 2.
MYOD knockout decreases muscle differentiation in vitro. (A) Deletion of MYOD genomic sequence causing MyoD protein deletion. The triangles indicate the primers used for genotyping. The sequence across the deletion junction is shown and was confirmed by sequencing the genotyping PCR product from the genomic DNA of MYOD−/− cells. (B) PCR products with the genotyping primers on genomic DNA confirmed MYOD sequence deletion in MYOD−/− cells. The products were sequenced to confirm the deletion junction shown in panel A. The complete absence of a WT genotype band in the MYOD−/− cells confirmed that no WT allele was left. (C) RNA-Seq confirmed deletion of all alleles of MyoD in the MYOD−/− cells. A Sashimi plot of the RNA-Seq reads shows that the deleted region (shaded) in exon 1 of the MYOD gene is missing in MYOD−/− cell RNA. (D) Western blot analysis confirms the absence of MyoD protein in MYOD−/− cells. Tubulin served as a loading control. (E) Immunofluorescence analysis of fixed cells 3 days after differentiation (DM3). The cells were immunostained with antibodies against MyoD and MHC. DAPI (4′,6-diamidino-2-phenylindole) was used to visualize nuclei. (F to H) qRT-PCR analysis of proliferating (GM) and differentiating (DM3) C2C12 cells that were WT or miR-1a-1−/−. Levels of MYOD, MYOGENIN, and MYH3 mRNAs normalized to GAPDH are shown relative to that in proliferating WT cells (WT GM). (I to L) qRT-PCR analysis of proliferating (GM) and differentiating (DM3) cells that were WT or MYOD−/−. Levels of the indicated RNAs normalized to GAPDH are shown relative to that in proliferating WT cells (WT GM). The values represent three biological replicates and are presented as means and SEM. Statistical significance was calculated using the Wilcoxon-Mann-Whitney test. *, P < 0.05.