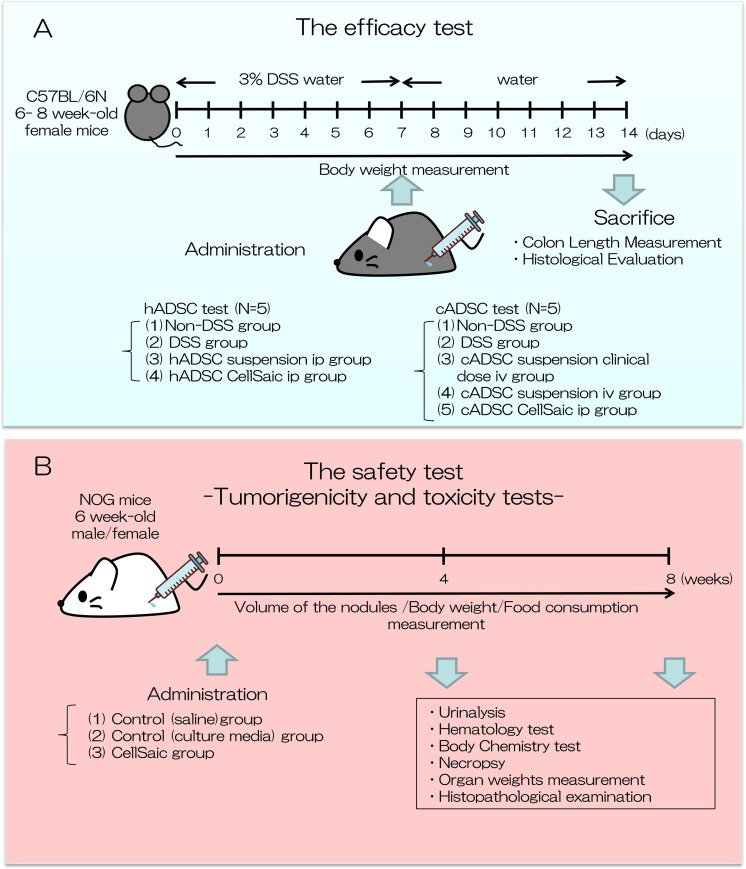
Fig. 1.
Experimental design of this study. We conducted an efficacy test and safety test of ADSC CellSaic. (A) Efficacy test: therapeutic effects of hADSC/cADSC CellSaic, evaluated in DSS-induced mouse model, compared with hADSC/cADSC suspension; Body weight was measured. Seven days after administration, mice were sacrificed, colon length was measured, and histological evaluation was conducted. (B) Safety test: Tumorigenicity and toxicity tests were conducted. cADSC CellSaic evaluated in NOG mouse. Volume of nodules, body weight, and food consumption were measured until 8 weeks. Four and 8 weeks after administration, urinalysis, hematology test, body chemistry test, necropsy organ weight measurement, and histopathological examination were conducted.