Abstract
Background
Despite its high prevalence, opioid-induced constipation (OIC) remains under-recognised and undertreated, and its true impact on wellbeing and quality of life (QoL) may be underestimated.
Methods
A quantitative, questionnaire-based international survey was conducted.
Results
Weak-opioid users appeared as bothered by constipation as strong-opioid users (38% vs 40%, respectively; p = 0.40), despite it causing less-severe physical symptoms and impact on QoL. Strong-opioid users meeting Rome IV OIC criteria appeared to experience greater symptomatic and biopsychosocial burden from constipation than those not satisfying these criteria. Almost one-fifth of respondents were dissatisfied with their current constipation treatment and around one-third found balancing the need for adequate pain relief with constipation side effects challenging. Consequently, more than half failed to adhere to their prescribed treatment regimens, or resorted to suboptimal strategies, e.g. 40% reduced their opioid intake, to relieve constipation. Almost 60% of healthcare professionals did not adequately counsel patients about constipation as a common side effect of opioid use.
Conclusions
Findings suggest that both weak- and strong-opioid users suffer comparable bother and decreased QoL, Rome IV criteria can identify patients with more-severe OIC, but may underdiagnose patients showing fewer symptoms, and increased education is needed to manage patients’ expectations and enable improved OIC self-management.
Keywords: Opioid-induced constipation, Rome IV criteria, patient survey, biopsychosocial burden, adherence, management and counselling
Key summary
1. Summarise the established knowledge on this subject
Although constipation is a common side effect of opioid use, even with the concomitant use of laxatives, opioid-induced constipation (OIC) remains under-recognised and undertreated.
There is evidence to suggest that the impact of OIC on patients’ overall wellbeing and quality of life may be underestimated by healthcare professionals.
The recently published Rome IV diagnostic criteria for OIC provide a valuable tool for use in clinical practice; however, data are awaited on the value of these new criteria in the assessment of patients with OIC in the real-life setting.
A better understanding of the unmet needs of patients with OIC would be valuable to improve the recognition and management of this condition.
2. What are the significant and/or new findings of this study?
Both weak- and strong-opioid users experience a considerable biopsychosocial burden caused by constipation, and the impact of OIC on users of weak opioids should not be underestimated.
Findings from this survey suggest that the new Rome IV criteria can identify patients with more severe OIC, but may underdiagnose patients with fewer/milder symptoms.
A substantial proportion of opioid users are not satisfied with their current constipation treatment and find balancing the need for adequate pain relief with constipation side effects challenging. This can lead to poor adherence with prescribed treatment regimens, or the use of suboptimal strategies to relieve constipation.
This survey highlighted a need for improved counselling for strong-opioid users, particularly regarding constipation as a potential side effect of opioid use.
Introduction
In recent years, the worldwide use of opioids has increased significantly.1 Constipation is a common side effect of opioid use, and can affect up to 81% of patients, even with the concomitant use of laxatives.2 Despite this, opioid-induced constipation (OIC) remains under-recognised and undertreated.3 Recently published Rome IV diagnostic guidance for functional gastrointestinal disorders now includes diagnostic criteria for OIC.4 While this is a much-needed step towards improving recognition of this condition, data are awaited on the value of these new criteria in the assessment of patients with OIC in real-life clinical practice.
OIC has a negative impact on patients’ wellbeing, affecting daily activities, work productivity and health-related quality of life,2 and is also associated with increased utilisation of healthcare resources.5 Available laxative therapies for OIC leave the patient with significant residual symptoms, which may lead them to adjust or stop their opioid intake in order to have a bowel movement, unless effectively counselled.3,6 The management of OIC is often hampered by factors including a lack of understanding and recognition among healthcare professionals (HCPs) of the potential morbidity associated with this condition. It is not clear whether there is any difference in the biopsychosocial disease burden of constipation in patients using either strong or weak opioids. There may also be a perception that strong-opioid use is associated with more severe side effects, and that these also occur at a higher frequency, compared with weak-opioid use, highlighting a need to further our understanding of the burden of OIC in users of strong or weak opioids.
Poor communication between HCPs and patients may be a further potential barrier to the effective management of OIC. Current guidance recommends that in addition to laxative use, first-line therapy should include non-pharmacological approaches, such as lifestyle modification and consumption of fibre-rich food. Therefore, good communication between the patient and HCP is key7–9 to encourage uptake and adherence to these measures. Studies in a variety of chronic disease conditions have demonstrated a link between effective patient engagement, management of functional symptoms and positive health outcomes.10 For example, an individualised self-care education programme, with and without peak flow monitoring, improved lung function in patients with asthma;11 patients on a group-based self-management programme for multiple sclerosis reported improvements in health-related quality of life;12 and disease-specific self-help groups were associated with improvements in self-reported general health status in patients with arthritis.13 It would be valuable to gain a better understanding of the information-seeking behaviour of patients with OIC, in order to determine the optimal approach to education and timely communication of appropriate information.
In an attempt to address some of the unmet needs in the management of OIC, this international survey was conducted to investigate whether long-term users of strong opioids (e.g. buprenorphine, fentanyl) with Rome IV-positive OIC differ in biopsychosocial disease burden vs those with constipation who do not satisfy the Rome IV criteria, and assess the impact of strong or weak (e.g. codeine, dihydrocodeine) opioids in patients with chronic pain who have constipation. The use of counselling resources, information seeking and sources of support in patients with constipation caused by the use of strong opioids was also explored.
Methodology
Two separate quantitative, questionnaire-based, online surveys were conducted by Insight Dojo, Guildford, United Kingdom (UK). One survey took place in France, Germany, Italy, Spain and the UK in respondents aged ≥40 years who were using strong opioids. A second survey was conducted simultaneously in Germany, Spain and the UK in respondents aged ≥18 years who were using weak opioids. Opioids were categorised according to whether they were less potent (‘weak opioids’) or were equipotent or more potent (‘strong opioids’) relative to morphine.14
Individuals were recruited for both surveys using two different types of online panel: (1) a large panel representative of the population in each market, and (2) targeted chronic pain panels, e.g. a ‘rheumatoid arthritis’ panel. All individuals who joined the panels consented to participate in the relevant online survey. Respondents had largely non-cancer-related chronic pain that was being managed with opioids, and OIC (defined as having ≤2 bowel movements per week, and expressing bother from constipation or dissatisfaction with current treatments for constipation). The International Chamber of Commerce/European Society for Opinion and Marketing Research International Code on Market, Opinion and Social Research and Data Analytics,15 and all local country codes of conduct for market research, were adhered to when conducting the surveys. All respondents received a very small incentive for taking part in the surveys, which ranged from €1.00 to 1.50 in France, Germany, Italy and Spain, and £0.75 to 1.00 in the UK. The survey questionnaires were developed by Insight Dojo in collaboration with Shionogi and assessed past medical history, opioid use, treatment and treatment-seeking behaviour, symptoms, burden of disease, and effects of constipation on quality of life (see Appendices 1 and 2). Respondents were not aware at the start of the surveys that they were about OIC. The surveys did not capture any self-reports of severity; rather, the analyses used objective measures of severity which were applied to self-reported symptoms and experiences.
To satisfy the Rome IV criteria for OIC, respondents who were users of strong opioids must have had new or worsening symptoms of constipation when initiating, changing or increasing opioid therapy plus two or more of the following symptoms defining functional constipation, with a frequency cut-off of 25%: straining, lumpy or hard stools, sensation of incomplete evacuation, sensation of anorectal blockage, use of manual manipulation to facilitate defaecation or <3 spontaneous bowel movements per week.16
To ensure comparability, only respondents aged ≥40 years from Germany, Spain and the UK were included in the analyses of strong- vs weak-opioid users. Analyses conducted to investigate approaches to the management of OIC, perceptions of treatment and counselling/information-seeking behaviour were conducted in the overall population of strong-opioid users.
Descriptive data were presented as proportions (%) of total subgroup populations, and Z tests were performed to establish significance between subgroups. A p value < 0.05 was defined as statistically significant.
Results
Analysis of Rome IV vs non-Rome IV subgroups of strong-opioid users
A total of 18,995 respondents from a nationally representative panel entered the survey from France, Germany, Italy, Spain and the UK, with 2016 eligible (i.e. overall strong-opioid population). Of these, 951 (47%) met the Rome IV diagnostic criteria for OIC.
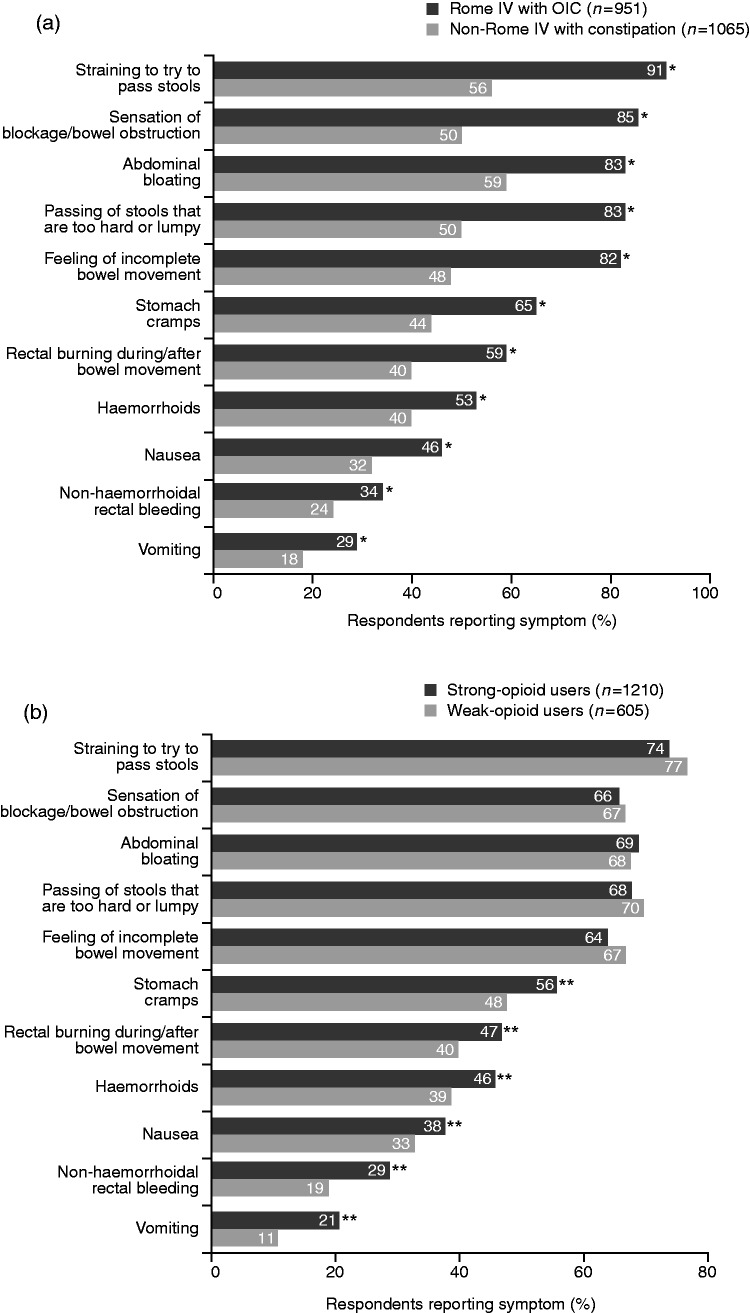
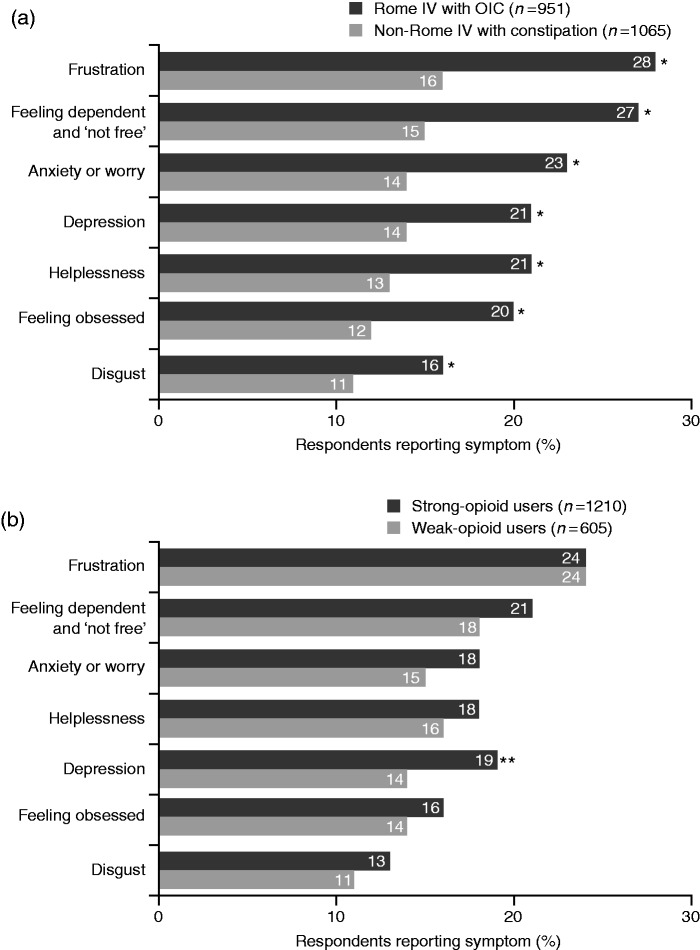
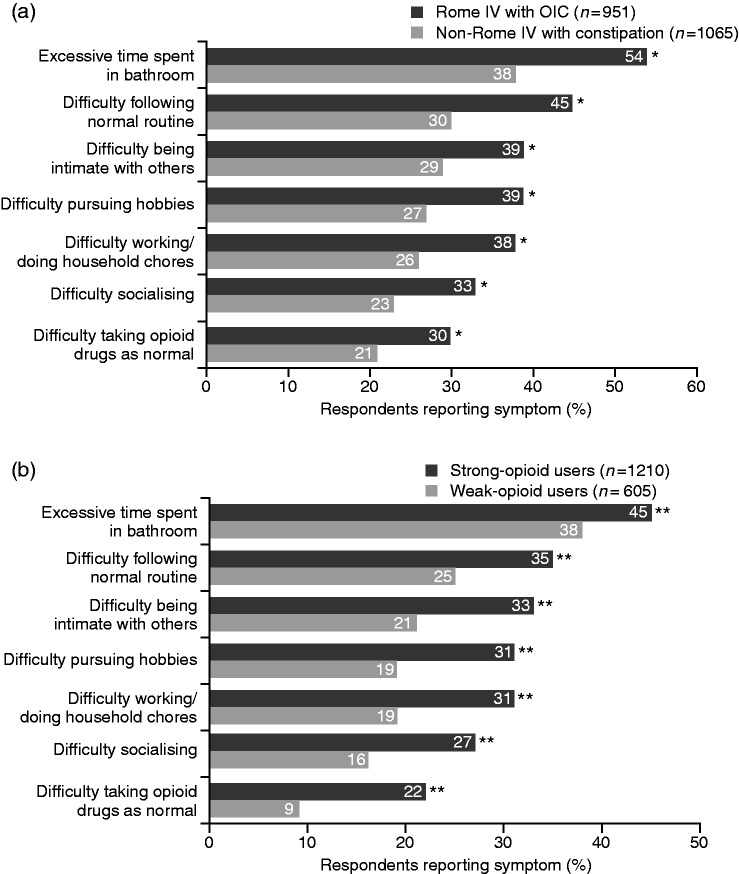
Baseline demographics and characteristics of the Rome IV OIC and non-Rome IV subgroups are shown in Table 1. Compared with non-Rome IV respondents, significantly more respondents who satisfied the Rome IV criteria reported physical effects of OIC, with the main symptoms being ‘straining to pass stools’, ‘abdominal bloating’ and ‘sensation of blockage/bowel obstruction’ (Figure 1(a)). A significantly greater proportion of Rome IV respondents felt emotional and psychological symptoms caused by OIC than did non-Rome IV respondents, with feelings of frustration, dependence and anxiety/worry being key (Figure 2(a)). While all respondents reported an impact of OIC on daily life/relationships, this was significantly greater among the Rome IV group members, who cited ‘excessive time spent in bathroom’, ‘difficulty following normal routine’ and ‘difficulty being intimate with others’ as key factors impacting on their wellbeing (Figure 3(a)).
Table 1.
Survey respondents’ baseline demographics and characteristics.
| Parameter, n (%) | Overall strong-opioid population (five countries) (N = 2016) | Strong-opioid population (three countries) (n = 1210) | Weak-opioid population (three countries) (n = 663) | Rome IV OIC subgroup (strong-opioid users) (n = 951) | Non-Rome IV with constipation subgroup (strong-opioid users) (n = 1065) |
|---|---|---|---|---|---|
| Age group, years | |||||
| 40–49 | 928 (46) | 544 (45) | 210 (35) | 443 (47) | 485 (46) |
| 50–59 | 713 (35) | 433 (36) | 218 (36) | 313 (33) | 400 (38) |
| 60–69 | 312 (15) | 194 (16) | 140 (23) | 158 (17) | 154 (14) |
| ≥70 | 63 (3) | 39 (3) | 37 (6) | 37 (4) | 26 (2) |
| Gender | |||||
| Male | 976 (48) | 576 (48) | 224 (37) | 467 (49) | 509 (48) |
| Female | 1040 (52) | 634 (52) | 381 (63) | 484 (51) | 556 (52) |
| Employment status | |||||
| Full-time/part-time/self-employed | 1443 (72) | 818 (68) | 324 (54) | 680 (72) | 763 (72) |
| Unemployed | 198 (10) | 125 (10) | 83 (14) | 81 (9) | 117 (11) |
| Semi-retired/retired | 372 (18) | 265 (22) | 198 (33) | 188 (20) | 184 (17) |
| Student/in full-time training | 3 (<1) | 2 (<1) | – | – | 1 (<1) |
| Single underlying condition that causes the most pain | |||||
| Chronic back pain | 586 (29) | 391 (32) | 160 (26) | 295 (31) | 291 (27) |
| Migraine | 342 (17) | 178 (15) | 43 (7) | 141 (15) | 201 (19) |
| Rheumatoid arthritis | 255 (13) | 155 (13) | 43 (7) | 136 (14) | 119 (11) |
| Osteoarthritis | 154 (8) | 60 (5) | 40 (7) | 80 (8) | 74 (7) |
| Fibromyalgia | 142 (7) | 109 (9) | 44 (7) | 63 (7) | 79 (7) |
| Joint pain | 101 (5) | 67 (6) | 76 (13) | 47 (5) | 54 (5) |
| Pain relating to cancer (not prostate cancer) | 34 (2) | 22 (2) | 4 (1) | 21 (2) | 13 (1) |
| Neuropathic pain related to diabetes | 24 (1) | 16 (1) | – | 8 (1) | 16 (2) |
| Other | 13 (<1) | 9 (<1) | 195 (32) | 5 (<1) | 8 (<1) |
| Duration of underlying condition | |||||
| >3 months but | 167 (8) | 87 (7) | 30 (5) | 85 (9) | 82 (8) |
| <1 year | 455 (23) | 223 (18) | 91 (15) | 224 (24) | 231 (22) |
| 1 to <3 years | |||||
| 3 to <5 years | 450 (22) | 253 (21) | 101 (17) | 207 (22) | 243 (23) |
| 5 to <10 years | 480 (24) | 312 (26) | 142 (23) | 221 (23) | 259 (24) |
| ≥10 years | 464 (23) | 335 (28) | 241 (40) | 214 (23) | 250 (23) |
| Current opioid use (multiple responses possible) | |||||
| Morphine | 683 (34) | 449 (37) | – | 324 (34) | 359 (34) |
| Fentanyl | 634 (31) | 253 (21) | – | 310 (33) | 324 (30) |
| Oxycodone | 576 (29) | 271 (22) | – | 294 (31) | 282 (26) |
| Oxycodone + naloxone | 273 (14) | 192 (16) | – | 118 (12) | 161 (15) |
| Buprenorphine | 213 (11) | 120 (10) | – | 91 (10) | 122 (11) |
| Tapentadol | 178 (9) | 108 (9) | – | 79 (8) | 99 (9) |
| Methadone | 159 (8) | 63 (5) | – | 78 (8) | 81 (8) |
| Meperidine | 137 (7) | 137 (11) | – | 62 (7) | 75 (7) |
| Hydromorphone | 131 (6) | 83 (7) | – | 61 (6) | 70 (7) |
| Diamorphine | 50 (2) | 50 (4) | – | 30 (3) | 20 (2) |
| Opium + acetaminophen | 37 (2) | – | – | 18 (2) | 19 (2) |
| Piritramide | 18 (1) | 18 (1) | – | 10 (1) | 8 (1) |
| Levomethadone | 16 (1) | 16 (1) | – | 7 (1) | 9 (1) |
| Co-codamol | – | – | 323 (53) | – | – |
| Tramadol | – | – | 236 (39) | – | – |
| Tilidine | – | – | 77 (13) | – | – |
| Tramadol + acetaminophen | – | – | 53 (9) | – | – |
| Dihydrocodeine | – | – | 34 (6) | – | – |
| Codeine | – | – | 33 (5) | – | – |
| Ketamine | – | – | 11 (2) | – | – |
| Pethidine | – | – | 14 (2) | – | – |
| Promethazine | – | – | <1 (1) | – | – |
| Current laxative use (multiple responses possible)a | |||||
| Stimulant laxative | 867 (43) | 470 (39) | 86 (14) | 434 (46) | 433 (41) |
| Osmotic agent | 830 (41) | 467 (39) | 92 (15) | 448 (47) | 382 (36) |
| Saline laxative | 322 (16) | 78 (6) | 19 (3) | 161 (17) | 161 (15) |
| Bulk-forming laxativeb | 146 (7) | 44 (4) | 7 (1) | 71 (7) | 75 (7) |
| Other (potentially non-laxative) | 125 (6) | 76 (6) | 9 (1) | 58 (6) | 67 (6) |
| Emollient laxative | 86 (4) | 76 (6) | 4 (1) | 36 (4) | 50 (5) |
| Lubricant laxative | 60 (3) | – | – | 30 (3) | 30 (3) |
| Combination of laxatives | 307 (15) | 264 (22) | 33 (5) | 140 (15) | 167 (16) |
| Guanylate cyclase 2C agonist | 20 (<1) | 16 (1) | 3 (<1) | 9 (1) | 11 (1) |
| Serotonin agonist | 9 (<1) | 7 (1) | – | 2 (<1) | 7 (1) |
| None | 254 (13) | 187 (15) | 10 (2) | 91 (10) | 163 (15) |
| Time of first opioid prescription | |||||
| In the last ≤3 months | 204 (10) | 8 (8) | 27 (4) | 101 (11) | 103 (10) |
| >3 months but <1 year | 432 (21) | 228 (19) | 61 (10) | 205 (22) | 227 (21) |
| 1 to <3 years | 632 (31) | 364 (30) | 150 (25) | 301 (32) | 331 (31) |
| 3 to <5 years | 359 (18) | 234 (19) | 125 (21) | 157 (17) | 202 (19) |
| ≥5 years | 389 (19) | 292 (24) | 242 (40) | 187 (20) | 202 (19) |
| Constipation prior to opioid use | |||||
| Much/slightly better | 1158 (57) | 654 (54) | 348 (58) | 951 (100) | 207 (19) |
| The same | 515 (26) | 322 (27) | 213 (35) | 0 (0) | 515 (48) |
| Much/slightly worse | 343 (17) | 234 (19) | 44 (7) | 0 (0) | 343 (32) |
Total percentages for each parameter may not equal 100 because of rounding.
Laxatives mentioned by respondents, and their classification into groups, are shown in Appendix 3. bAlso known as fibre supplements, e.g. psyllium/ispaghula husk, methylcellulose and sterculia.
OIC: opioid-induced constipation.
Figure 1.
Physical symptoms of constipation experienced by patients using opioids, stratified by Rome IV with OIC vs non-Rome IV with constipation (a) and weak- vs strong-opioid use (b) subgroups.
*p < 0.05 vs non-Rome IV with constipation subgroup. **p < 0.02 vs weak-opioid users.
OIC: opioid-induced constipation.
Figure 2.
Emotional and psychological symptoms of constipation experienced by patients using opioids, stratified by Rome IV with OIC vs non-Rome IV with constipation (a) and weak- vs strong-opioid use (b) subgroups.
*p < 0.05 vs non-Rome IV with constipation subgroup. **p < 0.05 vs weak-opioid users.
OIC: opioid-induced constipation.
Figure 3.
Impact of constipation on quality of life and social interactions experienced by patients using opioids, stratified by Rome IV with OIC vs non-Rome IV with constipation (a) and weak- vs strong-opioid use (b) subgroups.
*p < 0.05 vs non-Rome IV with constipation subgroup. **p < 0.05 vs weak-opioid users.
OIC: opioid-induced constipation.
Overall, significantly more Rome IV than non-Rome IV respondents experienced quite a lot/a great deal of bother from their OIC symptoms (42% vs 31%; p < 0.0001).
Analysis of strong- vs weak-opioid users
For the strong-opioid population, a total of 13,641 respondents from a nationally representative panel entered the survey from Germany, Spain and the UK, with 545 eligible (equating to a response rate of 4.0%). The remaining 665 respondents for the strong-opioid analysis were sourced from a target panel. For the weak-opioid population, 3856 respondents from a nationally representative panel entered the survey from Germany, Spain and the UK, with 663 included in the final analyses (for a response rate of 17.2%).
Baseline demographics and characteristics of the populations of strong- (n = 1210) and weak- (n = 663) opioid users aged ≥40 years are shown in Table 1. Compared with the weak-opioid population, more respondents in the strong-opioid population were male and younger. The majority of strong-opioid users were taking opioids for chronic back pain, while weak-opioid users had a variety of reasons for requiring pain relief. In general, strong-opioid users had started taking opioids more recently than weak-opioid users.
A comparable degree of bother from constipation symptoms was felt both by weak- and strong-opioid users, with 38% and 40% of respondents, respectively, stating that constipation bothered them quite a lot/a great deal (between-group comparison, p = 0.40). This pattern was reflected in the main psychological symptoms experienced due to constipation (i.e. frustration, dependence, anxiety) (Figure 2(b)).
The incidence of more-common physical symptoms (i.e. straining to pass stools, abdominal bloating, sensation of bowel blockage/obstruction) was similar in the strong- and weak-opioid groups. However, weak-opioid users experienced significantly fewer less-common physical symptoms of constipation compared with strong-opioid users, including stomach cramps, rectal burning and haemorrhoids (all p < 0.02) (Figure 1(b)). Moreover, the impact of constipation on quality of life/social symptoms was felt significantly less by weak-opioid users compared with users of strong opioids (Figure 3(b)).
Approaches to the management of OIC and perceptions of treatment in strong-opioid users
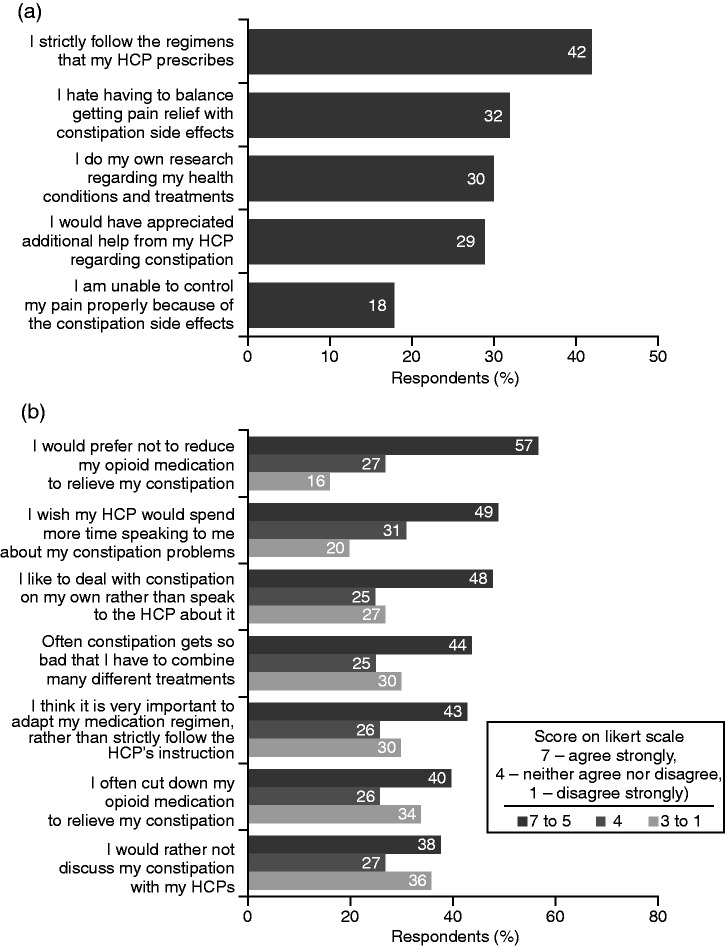
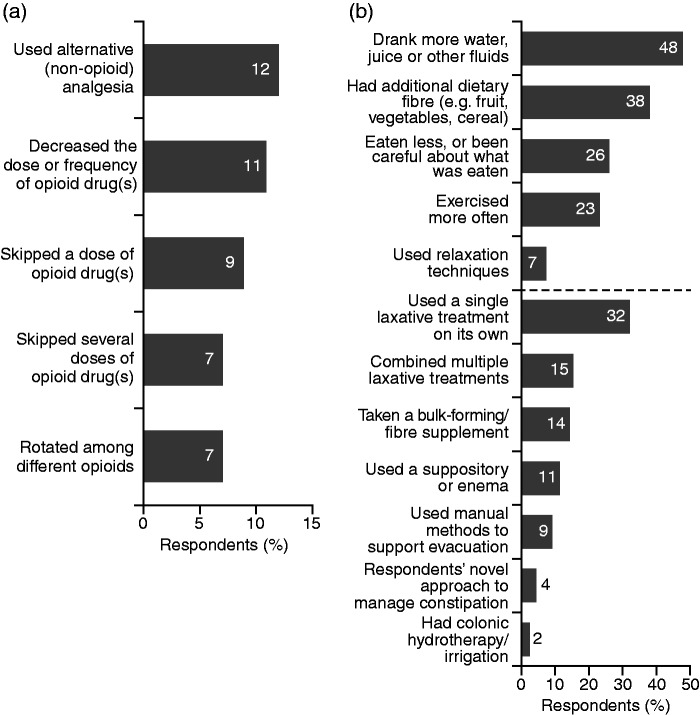
Around one-third of respondents found it difficult to combine management of pain relief with constipation symptoms and disliked having to balance between them (Figure 4(a)). Nearly one-fifth were very or somewhat dissatisfied with the effectiveness of their current constipation treatment, and only two-fifths strictly adhered to prescribed treatment regimens, with a substantial proportion researching other treatment options (Figure 4(a)). More than two-fifths of respondents admitted that their constipation becomes so bothersome that they have to combine different methods to relieve it, often cutting down their opioid medication (Figure 4(b)) or even skipping it entirely to relieve constipation (Figure 5(a)). This is despite more than half stating they would prefer not to reduce their opioid medication, if possible (Figure 4(b)). To manage their constipation, respondents regularly used a variety of approaches, including dietary measures, exercise and single or multiple laxative treatments (Figure 5(b)).
Figure 4.
Respondents’ approaches to the management of constipation and perceptions of treatment (strong-opioid users; N = 2016).
HCP: healthcare professional.
Figure 5.
Analgesia-related (a) and other (b) approaches used regularly by respondents to manage their constipation (strong-opioid users; N = 2016).
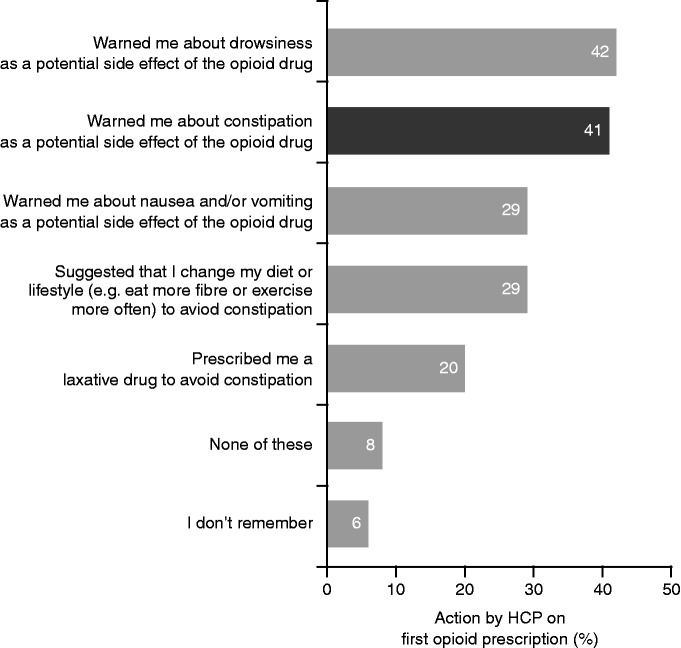
Counselling and information-seeking behaviour among strong-opioid users
Only two-fifths of respondents reported that their HCPs had warned them about constipation as a potential side effect of opioid use (Figure 6). Almost two-thirds (64%) reported that their HCP was the main information source on OIC. Other common sources of information were online search engines (45%), health forums (28%), blogs (12%) and other online resources (8%), as well as leaflets in their HCP’s workplace (21%), television (20%) and newspapers/magazines (19%). Respondents also sometimes received advice from their partners, friends and family members (cited by 16%, 16% and 14% of respondents, respectively). Although 49% of respondents stated that they would have liked their HCP to provide more information about OIC, a similar proportion preferred to deal with constipation on their own rather than discuss it with their HCP (Figure 4(b)).
Figure 6.
Counselling provided by HCPs when prescribing an opioid to respondents (strong-opioid users; N = 2016).
HCPs: healthcare professionals.
Discussion and conclusions
Despite its high prevalence among opioid users, OIC remains under-recognised and undertreated, and its true impact on patients’ overall wellbeing and quality of life may be underestimated. This survey found that users of both weak and strong opioids experience a considerable biopsychosocial burden caused by constipation. Subjectively, weak-opioid users appear to be as bothered by their constipation as strong-opioid users, despite it causing less-severe physical symptoms and a less-drastic impact on quality of life. This may reflect strong-opioid users having more serious underlying pain conditions and/or requiring other concomitant therapies, which may cause debilitating side effects of greater concern than constipation, compared with weak-opioid users.
To our knowledge, the present analysis is the first time that the Rome IV criteria for OIC have been evaluated in a real-world setting. Findings showed that compared with strong-opioid users who did not satisfy the Rome IV criteria, those who did meet Rome IV criteria appeared to experience greater symptomatic and biopsychosocial burden from their constipation. This suggests that Rome IV criteria can identify patients with more severe OIC, but may underdiagnose patients with constipation who do not demonstrate the full scale of symptoms. Further investigation of these preliminary findings is needed.
Overall, 18% of strong-opioid users who participated in this survey are not satisfied with their current constipation treatment and 32% report that they find it challenging to balance the need for adequate pain relief with constipation side effects. Consequently, many fail to adhere to their prescribed treatment regimens, or resort to using suboptimal strategies, such as reducing their opioid intake, to relieve constipation. Similarly, patients with cancer pain using opioids frequently experience burdensome constipation.17 Poor adherence with opioid analgesic regimens has been reported in 49%–70% of patients,18 with some stating that they would rather endure pain than experience the constipation associated with their opioid treatment.17
Opioids are prescribed for the management of pain by different types of clinicians across both primary and secondary care, and approaches to counselling and follow-up of patients may be very different. Research shows that patients who understand more about their disease often have improved health outcomes and use fewer healthcare resources. These benefits are even greater when patients are empowered and feel responsible for self-managing their condition.19 The initial contact with an HCP provides an opportunity for patients to ask questions, while also allowing the HCP to identify the patient’s individual needs in terms of education.20 This may be influenced by the patient’s perceptions, expectations and concerns, as well as factors affecting adherence with the prescribed treatment.21 This survey highlighted that patients’ expectations with regard to the provision of information are not being met, with almost half stating that they would have liked their HCP to provide more information about OIC. Advice and information should be tailored appropriately for patients initiating opioids vs those already established on treatment, and should address all biopsychosocial aspects of the burden of OIC.
While most respondents would like more support from their HCP, a substantial proportion prefer to deal with constipation on their own, perhaps owing to embarrassment or resignation to the symptoms of OIC. Previous research has found that more than one-third of patients do not raise the subject of OIC with their HCP,22 and one-fifth feel uncomfortable talking about their condition with an HCP, most often because of embarrassment.23 Therefore, HCPs should proactively raise the topic of OIC rather than wait for patients to initiate discussions, and should be attentive to how patients express the physical, psychological and practical impact of OIC.5
A recent observational study showed that drug safety is a major focus of patients who are prescribed new medicines for the long-term treatment of chronic conditions. This is particularly important given that a substantial proportion of patients cite safety issues as a reason for discontinuing treatment.21 The present survey found that many HCPs are not counselling patients adequately about constipation as a common potential side effect of opioid use, highlighting the need for increased education to manage patients’ expectations and enable improved self-management of their condition. The presentation of repeat-opioid users at pharmacies may provide a valuable opportunity for patient engagement, reinforcing information provided by the physician, and for counselling patients who are non-adherent with optimal treatment regimens.24
Digital technologies are increasingly being used to support patient care in the management of chronic diseases such as asthma, chronic obstructive pulmonary disorder, diabetes, heart failure and hypertension. They can provide education to improve self-management, enable monitoring, and facilitate contact with HCPs (e.g. via telephone support and follow-up).25 In this survey, patients reported using online search engines and online health forums as common sources of information, suggesting this group may be an appropriate target for digital educational interventions.
This survey had several limitations. Information on educational status and on the opioid doses used by the survey participants was not recorded. The information provided by participants was self-reported and was not verified from medical records or by their HCP. As such, it may have been subject to recall bias. It may be argued that patients with more severe OIC symptoms are more likely to respond to questionnaire requests. There is also a possibility that patients unhappy with their OIC treatment may have been more motivated to participate in the survey than those who were satisfied, which may lead to recruitment bias. However, respondents were not aware at the start of the questionnaire that it was about OIC. Any potential impact of the strong- and weak-opioid users being recruited via two different surveys is not known. Panel respondents were offered a financial incentive to participate in the survey; however, given the small monetary amount, it is not expected that this would be associated with any significant bias.
In conclusion, the increasing use of opioids globally means that a growing number of patients will experience OIC, driving the need for improved recognition and management of this condition. HCPs should not underestimate the morbidity associated with OIC, in particular the degree of bother caused by constipation in users of weak opioids, which is comparable to that of strong-opioid users. Approaches to counselling should be tailored to the individual patient’s needs and preferences, and should include education on constipation as a common potential side effect of opioid use and how this can be effectively managed, as well as addressing the biopsychosocial aspects of the burden of constipation. The recently published Rome IV diagnostic criteria provide a valuable tool for use in clinical practice. However, findings from this survey suggest that these criteria may be more effective in diagnosing patients with severe OIC, compared with those showing fewer symptoms.
Supplemental Material
Supplemental Material for The patient burden of opioid-induced constipation: New insights from a large, multinational survey in five European countries by Viola Andresen, Vivek Banerji, Genevieve Hall, Amir Lass and Anton V Emmanuel in United European Gastroenterology Journal
Acknowledgements
Editorial support in the development of this manuscript was provided by James Reed, PhD, of Blue Heeler Ltd, and funded by Shionogi in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Declaration of conflicts of interest
V Andresen has received speaker and/or consulting fees from Allergan, AstraZeneca, Boehringer Ingelheim, Falk, Ferring, KyowaKirin, Nordmark and Shionogi. V Banerji and G Hall are Insight Dojo employees. A Lass was contracted to Shionogi Limited at the time of the survey. AV Emmanuel has received honoraria for consultancy activities from Allergan, Almirall, AstraZeneca, Coloplast, Danone, GSK, Janssen, Medtronic, Mundipharma/Napp Pharmaceuticals, Norgine, Takeda and Wellspect; for advisory board participation from Allergan, Almirall, Coloplast, Danone, Ferring, Mundipharma/Napp Pharmaceuticals, Reckitt-Benckiser, Shire, Shionogi, Takeda and Wellspect; and has received research contracts from Coloplast, Ferring, GSK and Shire.
Funding
This work was supported by Shionogi.
Informed consent
All individuals who joined the panels consented to participate in the relevant online survey.
Ethics approval
Approval was not required as this was a survey.
References
- 1.O’Brien T, Christrup LL, Drewes AM, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain 2017; 21: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster LR. Opioid-induced constipation. Pain Med 2015; 16(Suppl 1): S16–S21. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A. Improving the recognition and diagnosis of opioid-induced constipation in clinical practice. J Fam Pract 2015; 64(10 Suppl 1): pii: jfp_6410l–pii: jfp_6410l. [PubMed] [Google Scholar]
- 4.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. Epub ahead of print 18 February 2016. DOI: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed]
- 5.Epstein RS, Teagarden JR, Cimen A, et al. When people with opioid-induced constipation speak: A patient survey. Adv Ther 2017; 34: 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LoCasale RJ, Datto C, Margolis MK, et al. Satisfaction with therapy among patients with chronic noncancer pain with opioid-induced constipation. J Manag Care Spec Pharm 2016; 22: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorn S, Lembo A, Cremonini F. Opioid-induced bowel dysfunction: Epidemiology, pathophysiology, diagnosis, and initial therapeutic approach. Am J Gastroenterol Suppl 2014; 2: 31–37. [DOI] [PubMed] [Google Scholar]
- 8.Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction – Recommendations of the Nordic Working Group. Scand J Pain 2016; 11: 111–122. [DOI] [PubMed] [Google Scholar]
- 9.Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: Pathophysiology, clinical consequences, and management. Gastroenterol Res Pract 2014; 2014: 141737–141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons LA, Wolever RQ, Bechard EM, et al. Patient engagement as a risk factor in personalized health care: A systematic review of the literature on chronic disease. Genome Med 2014; 6: 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang TT, Li YT, Wang CH. Individualized programme to promote self-care among older adults with asthma: Randomized controlled trial. J Adv Nurs 2009; 65: 348–358. [DOI] [PubMed] [Google Scholar]
- 12.Barlow J, Turner A, Edwards R, et al. A randomised controlled trial of lay-led self-management for people with multiple sclerosis. Patient Educ Couns 2009; 77: 81–89. [DOI] [PubMed] [Google Scholar]
- 13.Goeppinger J, Armstrong B, Schwartz T, et al. Self-management education for persons with arthritis: Managing comorbidity and eliminating health disparities. Arthritis Rheum 2007; 57: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 14.The Royal College of Anaesthetists. Dose equivalent and changing opioids, https://www.rcoa.ac.uk/faculty-of-pain-medicine/opioids-aware/structured-approach-to-prescribing/dose-equivalents-and-changing-opioids (2018, accessed May 2018).
- 15.International Chamber of Commerce/European Society for Opinion and Marketing Research (ICC/ESOMAR). International code on market, opinion and social research and data analytics, https://www.esomar.org/uploads/public/knowledge-and-standards/codes-and-guidelines/ICCESOMAR_Code_English_.pdf (2016, accessed March 2018).
- 16.Simrén M, Palsson OS, Whitehead WE. Update on Rome IV criteria for colorectal disorders: Implications for clinical practice. Curr Gastroenterol Rep 2017; 19: 15–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyne KS, Sexton C, LoCasale RJ, et al. Opioid-induced constipation among a convenience sample of patients with cancer pain. Front Oncol 2016; 6: 131–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen LM, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage 2013; 45: 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talboom-Kamp EP, Verdijk NA, Harmans LM, et al. An eHealth platform to manage chronic disease in primary care: An innovative approach. Interact J Med Res 2016; 5: e5–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graarup J, Ferrari P, Howard LS. Patient engagement and self-management in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vučićević KM, Miljković BR, Golubović BC, et al. Expectations, concerns, and needs of patients who start drugs for chronic conditions. A prospective observational study among community pharmacies in Serbia. Eur J Gen Pract 2018; 24: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coyne KS, LoCasale RJ, Datto CJ, et al. Opioid-induced constipation in patients with chronic noncancer pain in the USA, Canada, Germany, and the UK: Descriptive analysis of baseline patient-reported outcomes and retrospective chart review. Clinicoecon Outcomes Res 2014; 6: 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauck RL, Hong KJ, North J. Opioid-induced constipation survey in patients with chronic noncancer pain. Pain Pract 2017; 17: 329–335. [DOI] [PubMed] [Google Scholar]
- 24.Spadea T, Brusa P, Gnavi R, et al. Community pharmacy; towards a new model [article in Italian]. Recenti Prog Med 2017; 108: 168–171. [DOI] [PubMed] [Google Scholar]
- 25.Wootton R. Twenty years of telemedicine in chronic disease management – an evidence synthesis. J Telemed Telecare 2012; 18: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The patient burden of opioid-induced constipation: New insights from a large, multinational survey in five European countries by Viola Andresen, Vivek Banerji, Genevieve Hall, Amir Lass and Anton V Emmanuel in United European Gastroenterology Journal